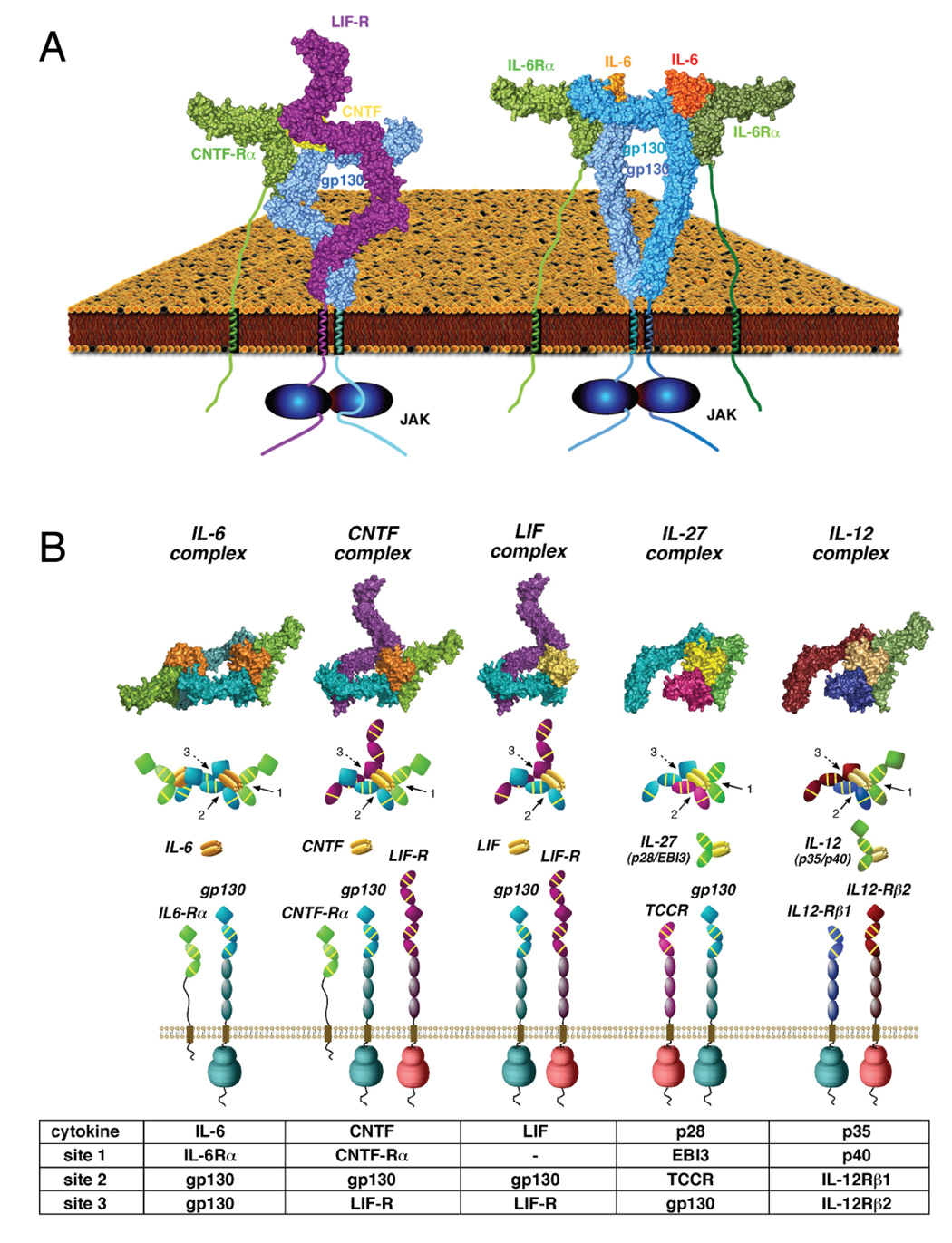
Figure 6. The organizing principles of the gp130 family of cytokine/receptor complexes.
(A) Structural comparison of homodimeric and heterodimeric gp130 receptor complexes. The structure to the left is the 1:1:1:1 gp130/LIF-R/CNTF-Rα/CNTF complex, and the image to the right is the 2:2:2 gp130/IL-6Rα/IL-6 complex (Skiniotis et al., 2005). In both cases, the bending of the ‘legs’ allows gp130 and LIF-R to form intimate receptor-receptor contact prior to entering the cell membrane, enforcing a close apposition of the receptor dimer. (B) Models were constructed for the cytokine-binding regions of representative classes of the gp130 ‘tall’ receptor family. gp130 homodimerization induced by IL-6 and IL-11 is represented by the structure of the gp130/IL-6Rα/IL-6 hexamer (panel 1). CNTF-induced heterodimerization of gp130 and LIF-R requires CNTF-Rα (which interacts with site 1), while LIF can heterodimerize gp130 and LIF-R in the absence of an alpha receptor. For IL-27, gp130 heterodimerizes with TCCR/WSX-1 (lacking IgD), with TCCR/WSX-1 binding to site 2, and gp130 interacting with site 3 on the p28 subunit. For the IL-12 cytokine receptor complex (panel 5), IL-12Rβ1 (lacking IgD) interacts with site 2 on the p35 subunit of the IL-12 heterodimer, while IL-12Rβ2 (contains IgD) interacts with site 3. All models were generated in COOT and PYMOL using known structures for gp130/IL-6Rα/IL-6 (PDB ID 1P9M), unliganded IL-6Rα (PDB ID 1N26), LIF-R/LIF (PDB ID 2Q7N), and unliganded LIF-R (PDB ID 3E0G).