Abstract
Background
IgG antibodies to pre-erythrocytic antigens are involved in prevention of infection and disease in animal models of malaria but have not been associated with protection against disease in human malaria.
Methods
Levels of IgG antibodies to circumsporozoite protein (CSP), liver-stage antigen type 1 (LSA-1), and thrombospondin-related adhesive protein (TRAP) were measured in 86 children in a malaria-holoendemic area of Kenya. The children were then monitored for episodes of clinical malaria for 52 weeks.
Results
Children with high levels of IgG antibodies to CSP, LSA-1, and TRAP had a decreased risk of clinical malaria (adjusted hazard ratio, 0.29; 95% confidence interval 0.10–0.81; P = .02), a lower incidence of clinical malaria (P = .006), protection from clinical malaria with a parasite level of ≥4000 parasites/μL (P = .03), and a higher hemoglobin level at enrollment (P = .009), compared with children with lower antibody levels. Protection against malaria morbidity was associated primarily with antibodies to CSP and LSA-1.
Conclusions
Kenyan children with high levels of IgG antibodies to the pre-erythrocytic antigens CSP, LSA-1, and TRAP have a lower risk of developing clinical malaria than children without high levels of these antibodies. The decreased risk of clinical malaria may be mediated in part by prevention of high-density parasitemia.
Antibody-mediated protection against human Plasmodium falciparum infection and disease was demonstrated in seminal studies in which infection and malaria-related symptoms cleared from infants with clinical P. falciparum malaria who received immunoglobulin obtained from African adults living in malaria-endemic areas [1]. Protection from malaria-related disease is thought to be due primarily to antibodies directed against blood-stage P. falciparum, since parasites from this stage, rather than those from pre-erythrocytic stages, underlie the pathogenesis of fever and other symptoms of malaria-associated illness. Although studies of pre-erythrocytic immunity in animals and humans have focused largely on T cell–mediated immunity [2-5], a role for antibodies to pre-erythrocytic antigens in protection from infection and disease in humans cannot be excluded. For example, antibodies to circumsporozoite protein (CSP) have been reported to impair the liver-based differentiation of sporozoites into merozoites in rodent malaria models [4, 6-9].
To date, no prospective longitudinal study conducted in a malaria-endemic population has demonstrated that the presence of antibodies to >1 pre-erythrocytic antigen is associated with protection against onset of clinical malaria. In a previous study of Kenyan adults, we demonstrated that high levels of IgG antibodies to CSP, liver-stage antigen type 1 (LSA-1), and thrombospondin-related adhesive protein (TRAP) were associated with relative protection against P. falciparum reinfection following cure of blood-stage infection with antimalarial drugs [10]. Greater protection was observed with pre-existing antibodies to all 3 antigens combined than with antibodies to a single antigen, and levels of antibodies to several blood-stage antigens did not increase or diminish the time to reinfection. Because the number of episodes of clinical malaria decreases with increasing age and cumulative exposure to P. falciparum in endemic areas, such as western Kenya, where malaria transmission is stable and high [11], this earlier study did not address the question of whether such antibodies might be associated with protection against malaria-attributable morbidity. Antibodies to pre-erythrocytic antigens could potentially decrease malaria morbidity by reducing the invasion of the liver by P. falciparum sporozoites or by impairing parasite development in the liver, leading to lower levels of parasitemia. Evidence of an association between antibodies to pre-erythrocytic antigens and protection from malaria morbidity would not prove that this relationship is causal, but it would support the notion that strong antibody responses to CSP and other pre-erythrocytic antigens contained in vaccines might serve as correlates of protective immunity in trials of vaccines containing CSP, TRAP, and/or LSA-1 [12-14]. To investigate these issues, we assessed whether high levels of antibodies to these antigens, both individually and together, were associated with protection from P. falciparum infection and symptomatic malaria in children in western Kenya.
SUBJECTS AND METHODS
Study site and participants
Written informed consent was obtained from parents or guardians of all participants. Ethical approval for the study was granted by the National Ethical Review Committee at Kenya Medical Research Institute (Nairobi, Kenya) and the Institutional Review Board for Human Studies at University Hospitals of Cleveland (Cleveland, OH) and Case Western Reserve University (Cleveland).
The study was conducted from August 2001 through July 2002 in the region of Kanyawegi in Nyanza province, Kenya [15]. A community meeting was held to describe the study, field assistants provided detailed information about the study to all households within the area, and parents and guardians interested in the study came to our collection sites to enroll their children. Inclusion criteria included age of >3 months and <8 years and permanent residence in the area. Exclusion criteria included acute or chronic illness, current symptoms of malaria, and use of antimalarial drugs within the previous 2 weeks. Study participants received no compensation but did receive free medical care for malaria.
Procedures
Approximately 0.5–1 mL of blood was collected at the beginning of the study. Samples were centrifuged, and plasma was removed and stored at -80°C for antibody testing. Ten μL of blood was obtained to measure the hemoglobin concentration, and blood smears were prepared to determine blood-stage infection by microscopy, as described elsewhere [15]. National policy at the time of study was that antimalarial treatment should not be administered to children with asymptomatic parasitemia, because the prevalence of asymptomatic parasitemia was very high and the receipt of drug treatment might affect development of clinical immunity. Thus, antimalarial drug treatment was not given at enrollment. However, all enrolled children who developed clinical malaria during the 52-week follow-up period were given antimalarial therapy (i.e., sulfadoxine-pyrimethamine; amodiaquine or quinine were administered to children allergic to sulfadoxine-pyrimethamine) recommended by the Kenya Ministry of Health.
Active surveillance for clinical malaria was conducted over a 52-week period after enrollment. Parents of children were told to contact their village-based field assistant if their child had a fever or chills. Village-based field assistants also asked weekly whether any participant had fever or chills. Children with fever or chills were seen by a clinical officer and treated with antimalarial drugs if they had P. falciparum on a blood smear. An episode of clinical malaria was defined as self-report of fever or chills or an axillary temperature of >37.5°C, plus asexual-stage P. falciparum on a blood smear. A second end point was defined as fever or chills plus an asexual-stage P. falciparum density of ≥4000 parasites/μL. This density was previously found to have the best overall sensitivity and specificity in this area of Kenya for fever in a large epidemiologic study of malaria morbidity in children aged 1–14 years [11].
Antibodies to CSP and LSA-1 were tested using the central repeat sequence peptides of these antigens to which individuals from malaria-endemic areas have previously been observed to have IgG antibodies. The (NANP)5 repeat peptide was used for CSP [16], and the LAKEKLQGQQSDLEQERLAKEKLQEQQSDLEQERLAKEKLQ (LSA-Rep) was used for LSA-1 [17]. Antibodies to TRAP were tested using recombinant P. falciparum TRAP (3D7) expressed in E. coli and provided by one of us (D.E.L.). The gene fragment encoding amino acids D48 to K394 was amplified from the 3D7 strain of parasite genomic DNA, using PCR with gene-specific sense and antisense primers.
Overall and IgG-subclass levels of antibodies were quantified by ELISA, as described elsewhere [10]. Antibody levels were expressed in arbitrary units (AU) and calculated by dividing the ODs for samples from study participants by the mean OD +3 standard deviations (SDs) for samples from 40 North Americans never exposed to malaria. Serum specimens obtained from 9 North Americans that had ODs representative of those of the 40 North American samples were tested on individual plates as controls. Control specimens were considered to be positive for antibodies if the antibody level was ≥1.0 AU. ODs for the AU cutoff (AU = 1.0) were 0.144 for IgG antibodies to CSP, 0.078 for antibodies to LSA-1, and 0.110 for antibodies to TRAP. On each plate, samples from study participants were tested along with antibody-positive control samples. The following mean ODs (SDs) were recorded for the control samples: 0.89 (0.52), for IgG antibodies to CSP; 1.39 (0.83), for LSA-1; and 0.56 (0.38), for TRAP.
Statistical analysis
To avoid statistical problems associated with use of multiple comparisons defined by different antibody threshold values, a single antibody threshold was set before analysis as the primary exposure variable. This threshold was defined as a high level (i.e., >2 AU) of IgG antibodies to all pre-erythrocytic antigens under study (i.e., CSP, LSA-1, and TRAP). This threshold was chosen because, in a previous study of adults from Kanyawegi, >2 AU of antibodies correlated with time to reinfection with blood-stage P. falciparum following cure of infection mediated by quinine and doxycycline therapy [10]. Secondary exposure variables were high levels of antibodies to individual antigens. The primary outcome variable was protection from clinical malaria, defined as protection from an episode of malaria during the study period. Secondary outcome variables were clinical malaria with a parasite level of ≥4000 parasites/μL, the incidence density of clinical malaria, and the hemoglobin level at the time of sample collection. Primary and secondary variables were chosen before the analysis. With this plan, P values for the primary and secondary end points were not adjusted for multiple comparisons.
Correlations between continuous variables (e.g., levels of antibodies to different antigens) were assessed by Spearman rank correlation analysis. Associations between categorical variables (e.g., presence of high levels of antibodies to specific antigens and clinical malaria) were assessed by χ2 analysis. Kaplan-Meier survival analysis and the log rank test were used to compare the times to development of P. falciparum parasitemia in study participants with and those without high levels of IgG antibodies to pre-erythrocytic antigens. The risk of the first symptomatic malaria episode was assessed with Cox proportional hazards regression modeling. The cumulative incidence of clinical malaria episodes was assessed with negative binomial regression analysis, in which all malaria episodes during the 52-week period were recorded. The Stata cluster option was used to obtain robust variance estimates that adjusted for within-person correlation of repeat malaria episodes. Age-adjusted difference in hemoglobin level at enrollment was assessed with multiple linear regression modeling. Age, bed net use, and malaria infection status at the time of study enrollment were adjusted for in all final models.
Sample size was determined by 2-sample comparison of proportions, with the following assumptions: of the children enrolled, ≥33.3% would have high levels of antibodies to all 3 pre-erythrocytic antigens; and ≥80% of children without high levels of antibodies would develop clinical malaria over the 52-week period of study. A sample size of 77 children was required to provide 90% power to detect a 50% reduction in clinical disease in children with high levels of antibodies. Statistical analysis was done with Stata, version 9.0 (Stata).
RESULTS
Study enrollment and follow-up
Eighty-six children (45 males and 41 females) were recruited; all were <8 years old (mean age [±SD], 5.26 ± 1.72 years). Sixty-eight children (79.1%) had P. falciparum parasitemia at the time of enrollment, but none were symptomatic. Only 3 children (3.5%) lived in a household where bed nets were used. Survival and Cox regression analyses were performed using data for all 86 participants. Data for 3 children who moved out of the study area during the follow-up period were censored in Cox regression analysis at the week in which they left (weeks 6, 17, and 33). Frequency comparisons were performed for the 83 children who were followed for the full study period.
IgG antibody levels and subclasses
Levels of IgG antibodies to pre-erythrocytic antigens were high (table 1) but lower than those reported previously for adults from the same area [10]. A total of 48.8% of children had high levels (i.e., >2 AU) of antibodies to CSP; 57.0%, to LSA-1; and 57.0%, to TRAP. IgG antibody levels or the presence of high levels of antibodies (AU > 2) to CSP, LSA-1, or TRAP did not differ in children with or without parasitemia at enrollment (data not shown). Levels of IgG antibody subclasses were checked in a subset of 28 children. Most antibodies belonged to the IgG1 and IgG3 subclasses (table 2), although IgG2 to LSA-1 and TRAP was seen in lower frequencies. The proportion of children with antibodies belonging to the various IgG subclasses was similar to the proportion reported previously for adults, with the exception of IgG1 antibody to TRAP (21.4% of children versus 85% of adults) and IgG2 antibody to LSA-1 (32.4% of children versus 0% of adults) [10]. Levels of antibody to CSP and to LSA-1 were strongly correlated (Spearman’s rho, 0.44; P < .001), whereas levels of antibodies to CSP and to TRAP (Spearman’s rho, 0.11; P = .30) and levels of antibodies to LSA-1 and to TRAP (Spearman’s rho, 0.20; P = .12) were not. Of the 3 children who moved out of the area, 1 had high levels of antibodies to all 3 antigens, and 2 did not.
Table 1.
Characteristics of IgG antibodies to circumsporozoite protein (CSP), liver-stage antigen type 1 (LSA-1), and thrombospondin-related adhesive protein (TRAP) in 86 Kenyan children.
| Antigen | Median AU (range) | >2 AU, % of children |
|---|---|---|
| CSP | 1.94 (0.12–21.4) | 48.8 |
| LSA-1 | 3.16 (0.01–61.6) | 57.0 |
| TRAP | 2.72 (0.29–29.8) | 57.0 |
NOTE. AU, arbitrary unit. See Subjects and Methods for a description of how AU was calculated.
Table 2.
Frequencies of high-level antibody responses to circumsporozoite protein (CSP), liver-stage antigen type 1 (LSA-1), and thrombospondin-related adhesive protein (TRAP) in 28 Kenyan children, by IgG subclass.
| Frequency of high-level antibody response by IgG subclass, % of children
|
||||
|---|---|---|---|---|
| Antigen | IgG1 | IgG | IgG3 | IgG4 |
| CSP | 71.4 | 0 | 60.7 | 0 |
|
| ||||
| LSA-1 | 57.1 | 32.1 | 85.7 | 0 |
|
| ||||
| TRAP | 21.4 | 7.1 | 89.2 | 0 |
IgG antibodies to individual antigens and protection from clinical malaria
Considering each of the antigens individually, a high level of IgG antibody to LSA-1 was correlated with protection from clinical malaria (P = .02) (table 3). A similar trend was seen with CSP (P = .16) but not with TRAP. Trends toward protection from malaria with a parasite level of ≥4000 parasites/μL were seen with all 3 antigens, but none reached statistical significance (P > .05 for all). Children who had a high level of antibody to LSA-1 had fewer episodes of malaria than children who did not (P = .02), and a similar trend was seen for antibody to CSP but not for antibody to TRAP. Finally, hemoglobin levels were significantly higher in children who had a high level of IgG antibody to CSP than in children who did not (P = .004).
Table 3.
Clinical malaria outcomes and hemoglobin (Hb) levels in Kenyan children with and those without high levels of IgG antibodies to circumsporozoite protein (CSP), liver-stage antigen type 1 (LSA-1), and thrombospondin-related adhesive protein (TRAP).
| Clinical malaria
|
Malaria with parasitemiac |
Cumulative malaria incidence
|
Age-adjusted Hb level
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Antigen, IgG level >2 AU | No. of children | Percentage of childrena | Pb | Percentage of children | Pb | Episodes/person-year | Pb | Mean g/dL ± SD | Pd |
| CSP | |||||||||
|
| |||||||||
| Yes | 39 | 43.6 | .16 | 12.8 | .24 | 1.00 | .13 | 8.94 (0.81) | .004 |
|
| |||||||||
| No | 44 | 59.1 | 22.7 | 1.36 | 8.13 (0.51) | ||||
|
| |||||||||
| LSA | |||||||||
|
| |||||||||
| Yes | 47 | 40.4 | .02 | 12.7 | .15 | 0.94 | .02 | 8.78 (0.84) | .14 |
|
| |||||||||
| No | 36 | 66.7 | 25.0 | 1.51 | 8.31 (0.53) | ||||
|
| |||||||||
| TRAP | |||||||||
|
| |||||||||
| Yes | 43 | 51.1 | .88 | 11.6 | .11 | 1.31 | .27 | 8.59 (0.85) | .77 |
|
| |||||||||
| No | 40 | 52.8 | 25.0 | 1.04 | 8.50 (0.53) | ||||
|
| |||||||||
| CSP, LSA-1, TRAP | |||||||||
|
| |||||||||
| Yes | 17 | 23.5 | .009 | 0 | .03 | 0.41 | <.001 | 9.29 (0.88) | .008 |
|
| |||||||||
| No | 66 | 59.1 | 22.7 | 1.39 | 8.27 (0.50) | ||||
NOTE. AU, arbitrary unit. See Subjects and Methods for a description of how AU was calculated.
Percentage of children who had >1 episode of clinical malaria.
By χ2 test (or Fisher’s exact test when appropriate).
Defined as a parasite level of >4000 parasites/μL.
By multiple linear regression analysis.
IgG antibodies to multiple antigens and protection from clinical malaria
Children who had high levels of antibodies to all 3 antigens had a decreased frequency of clinical malaria, compared with children with high levels of antibodies to <3 of the antigens (23.5% vs. 59.1%; P = .009) (table 3). The corresponding Kaplan-Meier curve is presented in figure 1. Children with high levels of IgG antibodies to CSP, LSA-1, and TRAP combined were also completely protected from episodes of clinical malaria with a parasite level of ≥4000 parasites/μL (0% vs. 22.7% episodes; P = .03), experienced fewer episodes of clinical malaria (0.39 vs. 1.37 episodes per person-year; P < .001), and had a higher age-adjusted hemoglobin level (9.29 g/dL vs. 8.27 g/dL; P = .008). For all outcomes, the differences were greater for children with high levels of antibodies to all 3 antigens combined than for children with a high level of antibody to a single antigen.
Figure 1.
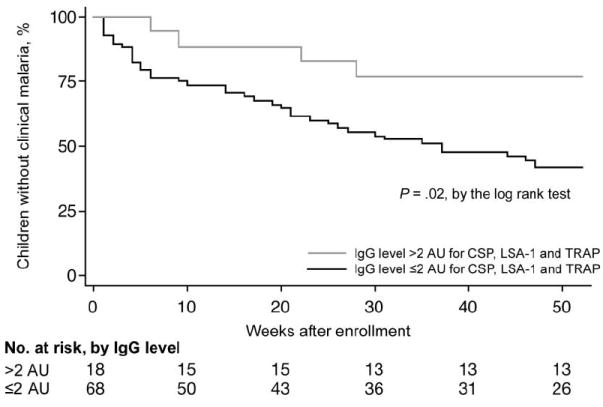
Risk of clinical malaria in children with and children without high levels of antibodies to circumsporozoite protein (CSP), liver-stage antigen type 1 (LSA-1), and thrombospondin-related adhesive protein (TRAP). A high level of antibody is defined as an antibody level of >2 arbitrary units (AU). See Subjects and Methods for a description of how AU was calculated.
The reduced risk and incidence of clinical malaria and the higher mean hemoglobin level in children with high levels of antibodies to CSP, LSA-1, and TRAP remained after adjustment for age, parasitemia at enrollment, and bed net use (table 4). Adjusted risk ratios for clinical malaria with a parasite level of ≥4000 parasites/μL could not be calculated because no child with high levels of antibodies to CSP, LSA-1, and TRAP developed this outcome during the study period. The reduced risk and incidence of clinical malaria and the elevated mean hemoglobin level in children with antibodies to CSP and LSA-1 alone were similar to those in children with antibodies to CSP, LSA-1, and TRAP (table 4). Post hoc exploratory analysis of cutoff levels of >2 AU for any antigen demonstrated that no levels >2 AU were associated with greater protection against malaria.
Table 4.
Adjusted differences in malaria-related clinical outcomes for Kenyan children with and those without high levels of IgG antibodies to circumsporozoite protein (CSP), liver-stage antigen type 1 (LSA-1), and thrombospondin-related adhesive protein (TRAP).
| Risk of clinical malaria
|
Cumulative incidence of malaria (multiple episodes)
|
Change in Hb level, g/dL
|
||||
|---|---|---|---|---|---|---|
| Antigens | HR (95% CI) | Pa | IRR (95% CI) | Pb | Mean difference (95% CI) | Pc |
| CSP, LSA-1, TRAP | 0.29 (0.10–0.81) | .02 | 0.29 (0.11–0.74) | .009 | +1.00 (+0.25 to +1.75) | .009 |
|
| ||||||
| CSP, LSA-1 | 0.43 (0.21–0.88) | .02 | 0.58 (0.31–1.08) | .09 | +1.07 (+0.45 to +1.68) | .001 |
NOTE. All values are adjusted for age, bed net use, and parasitemia at the time of enrollment. CI, confidence interval; HR, hazard ratio; IRR, incidence rate ratio.
By Cox regression analysis.
By negative binomial regression analysis.
By multiple linear regression analysis.
DISCUSSION
We previously examined adults from the same area of Kenya and reported that high levels of IgG antibodies to CSP, LSA-1, and TRAP, primarily those of the IgG1 and IgG3 subclasses, were associated with protection against P. falciparum infection [10]. In the present study, we demonstrated that high levels of IgG antibodies to the pre-erythrocytic antigens CSP, LSA-1, and TRAP were strongly associated with a reduction in the risk of multiple related but distinct outcomes of P. falciparum infection in Kenyan children, including the first episode of clinical malaria, the presence of clinical malaria with a parasite level of ≥4000 parasites/μL, the cumulative incidence of clinical malaria, and a decreased hemoglobin level. High levels of antibodies to all 3 antigens were more strongly associated with protection from symptomatic malaria than were high levels of antibodies to individual antigens. The present study is the first to examine prospectively the association between high levels of antibodies to multiple pre-erythrocytic antigens and protection from disease in African children naturally exposed to P. falciparum. Our findings suggest that high levels of antibodies to CSP, LSA-1, and TRAP may be useful immune correlates of protection against P. falciparum infection and disease in children from malaria-endemic areas of Africa. The data provide an impetus for further studies of this relationship in other malaria-endemic areas where vaccines will be tested and administered to infants and children.
In vitro and in vivo studies of rodent malaria models demonstrate that antibodies to CSP or TRAP impair or prevent infection [18-21], providing biological plausibility for the idea that antibodies to these antigens may play a role in protection from infection and disease. Human and mouse studies also strongly support CD4+ and CD8+ T cell interferon-γ responses as important in protection against liver-stage malaria [5, 15, 22-24]. Our unpublished data show that cytokine and antibody responses to LSA-1 and TRAP do not correlate with each other, suggesting that antibody levels are not a marker for interferon-γ responses but may impair directly or indirectly the progression of P. falciparum from the sporozoite stage to the blood stage. A potential mechanism for the additive effect of antibodies with different specificities is that each acts at a different step of the parasite’s life cycle. CSP is important in adhesion of the sporozoite to the basolateral membrane of the hepatocyte [25], and monoclonal antibodies to CSP inhibit parasite invasion of hepatoma cells [6]. TRAP is essential for sporozoite gliding motility [26] and hepatocyte invasion [21], and anti-TRAP antibodies have been shown to prevent sporozoite invasion of hepatocytes [20, 21]. Antibodies to LSA-1 may increase antibody-dependent cellular inhibition of parasite growth or increase clearance of liver-stage merozoites before their release into the bloodstream [27]. Recent work indicates that IgG1 and IgG3 antibodies—the major subclasses observed in our study (table 2)—mediate this type of growth inhibition [28, 29].
Among the 3 pre-erythrocytic antigens we studied, the presence of antibody to LSA-1 was most strongly associated with protection from clinical malaria and decreased episodes of clinical malaria (table 4). LSA-1 is expressed exclusively by liver-stage P. falciparum (no mouse malarial orthologue has been described), and antibody to the LSA-1 central repeat peptide has been shown in one [30] but not another [31] study to be associated with protection from clinical malaria. The differences in association may be attributable to transmission differences or to differences in the levels of antibody to LSA-1 achieved in these populations. In the present study, most children had antibody to LSA-1, and only the presence of high levels of antibodies (in contrast to the presence of antibodies at any level) was associated with protection from clinical malaria. Antibody to CSP has not been proven to be correlated with protection against infection or clinical malaria in individuals in malaria-endemic areas in prior studies [32-34]. In contrast, recent studies of the CSP-based RTS,S/AS02 vaccine given to Mozambican children aged 1–4 years showed efficacies of 29.9% and 28.9% against clinical malaria, defined as the presence of fever (temperature, ≥37.5°C) and parasitemia (parasite level, >2500 parasites/μL), 6 and 21 months, respectively, after vaccination [35, 36]. Levels of antibodies to recombinant CSP containing a segment of the repeat region in the RTS,S vaccine were far higher in the vaccine group, compared with those in the control group. Vaccinated children with the highest tertile of anti-CSP antibody titers did not differ from those with the lowest tertiles with respect to the risk of developing clinical malaria, but the levels in children with the lowest tertile were still many times higher than those in naturally exposed children. It is possible that antibodies to the repeat NANP peptide and the recombinant CSP molecule used in the RTS,S vaccine may not be concordant under various conditions of natural exposure to malaria. It may also be that the “cutoff value” for protection associated with antibodies to CSP is higher than the standard cutoff value that distinguishes between the presence and the absence of antibodies but is lower than the very high levels attained with the RTS,S vaccine. In the present study of childhood malaria and in our previous report assessing P. falciparum infection in adults [10], nonsignificant trends toward protection were seen in children with a high level of antibody to CSP, which supports a possible effect of anti-CSP antibody on both P. falciparum infection and disease in children exposed to areas where the rate of malaria transmission is high, such as western Kenya. It is unlikely that antibody-associated protection was due to a general “boost” in immunity from a single recent infection, since children with and children without parasitemia at the time of enrollment had similar antibody levels and risks of disease. It is possible that high levels of antibodies are markers for other as yet undefined protective mechanisms associated with cumulative exposure to infection, but use of these factors as correlates of protective immunity remains valid even if this is the case.
Immune responses such as production of antibodies to pre-erythrocytic antigens may not completely prevent blood-stage infection but may control the parasite density and the clonality of parasitemia by reducing the invasion of P. falciparum sporozoites into the liver or by impairing progressive development of parasites in the liver. Previous data demonstrating increased levels of anti-CSP antibodies [10] and decreasing parasite densities [37] in asymptomatic adults in a malaria-holoendemic area support this notion. Protection from higher-density parasitemia reported here supports this idea—none of the 18 children with high levels of antibodies to CSP, LSA-1, and TRAP developed a parasite level of ≥4000 parasites/μL during their episode of clinical malaria. Protection from high-density parasitemia may be a pathway through which partial protection against pre-erythrocytic stages of P. falciparum reduces the burden of malaria-related clinical illness, as lower density parasitemia may more readily be controlled by other mechanisms of anti-disease immunity, such as antibodies directed against variant malaria antigens expressed on the surface of infected erythrocytes. Lower density parasitemia may also result in a decrease in hemolysis and/or associated bone marrow suppression, resulting in higher hemoglobin levels.
The findings presented here raise issues relevant to the evaluation and use of malaria vaccines. The degree of natural exposure to malaria required to attain high levels of IgG antibodies to CSP, TRAP, and LSA-1 is not known. In malaria-endemic areas with transmission rates lower than those in western Kenya, children may not attain the levels reported here. In addition, the reason why levels of antibodies to the different pre-erythrocytic antigens are heterogeneous and why only some children develop high levels of antibodies to all 3 antigens are not known. Differences in exposure to infective sporozoites at the household level, as well as genetic factors, may contribute.
Finally, results of our study lend support to the goal of developing a multiple-antigen subunit or attenuated whole parasite vaccine that includes pre-erythrocytic antigens as a target. If multiple-antigen vaccines can induce higher levels of antibodies and T effector cells than levels induced by natural infection or a single-antigen vaccine, they may improve the strength of protection from malaria-related morbidity provided by these exposures.
Acknowledgments
We thank the field assistants, microscopists, and clinical officers involved in the study, as well as the participating children and their parents and guardians. We also thank Daniel Pregibon, for his work on antibody testing, and Daniel Tisch, for additional review of statistical analysis.
Financial support: National Institute of Allergy and Infectious Diseases (grant AI43906 to J.W.K. and grant AI01572 to C.C.J.).
Footnotes
Potential conflicts of interest: none reported.
This study is published with the permission of the Office of the Director of the Kenya Medical Research Institute.
References
- 1.Cohen S, McGregor IA, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–7. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 2.John CC, Sumba PO, Ouma JH, Nahlen BL, King CL, Kazura JW. Cytokine responses to Plasmodium falciparum liver-stage antigen 1 vary in rainy and dry seasons in highland Kenya. Infect Immun. 2000;68:5198–204. doi: 10.1128/iai.68.9.5198-5204.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reece WH, Pinder M, Gothard PK, et al. A CD4+ T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat Med. 2004;10:406–10. doi: 10.1038/nm1009. [DOI] [PubMed] [Google Scholar]
- 4.Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330:664–6. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 5.Doolan DL, Hoffman SL. The complexity of protective immunity against liver-stage malaria. J Immunol. 2000;165:1453–62. doi: 10.4049/jimmunol.165.3.1453. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee S, Wery M, Sharma P, Chauhan VS. A conserved peptide sequence of the Plasmodium falciparum circumsporozoite protein and antipeptide antibodies inhibit Plasmodium berghei sporozoite invasion of Hep-G2 cells and protect immunized mice against P. berghei sporozoite challenge. Infect Immun. 1995;63:4375–81. doi: 10.1128/iai.63.11.4375-4381.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodrigues M, Nussenzweig RS, Zavala F. The relative contribution of antibodies, CD4+ and CD8+ T cells to sporozoite-induced protection against malaria. Immunology. 1993;80:1–5. [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart MJ, Nawrot RJ, Schulman S, Vanderberg JP. Plasmodium berghei sporozoite invasion is blocked in vitro by sporozoite-immobilizing antibodies. Infect Immun. 1986;51:859–64. doi: 10.1128/iai.51.3.859-864.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang R, Charoenvit Y, Corradin G, et al. Induction of protective polyclonal antibodies by immunization with a Plasmodium yoelii circumsporozoite protein multiple antigen peptide vaccine. J Immunol. 1995;154:2784–93. [PubMed] [Google Scholar]
- 10.John CC, Moormann AM, Pregibon DC, et al. Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. Am J Trop Med Hyg. 2005;73:222–8. [PubMed] [Google Scholar]
- 11.Bloland PB, Boriga DA, Ruebush TK, et al. Longitudinal cohort study of the epidemiology of malaria infections in an area of intense malaria transmission. II. Descriptive epidemiology of malaria infection and disease among children. Am J Trop Med Hyg. 1999;60:641–8. doi: 10.4269/ajtmh.1999.60.641. [DOI] [PubMed] [Google Scholar]
- 12.Alonso PL. Malaria: deploying a candidate vaccine (RTS,S/AS02A) for an old scourge of humankind. Int Microbiol. 2006;9:83–93. [PubMed] [Google Scholar]
- 13.Moorthy VS, McConkey S, Roberts M, et al. Safety of DNA and modified vaccinia virus Ankara vaccines against liver-stage P. falciparum malaria in non-immune volunteers. Vaccine. 2003;21:1995–2002. doi: 10.1016/s0264-410x(02)00771-5. [DOI] [PubMed] [Google Scholar]
- 14.Prieur E, Gilbert SC, Schneider J, et al. A Plasmodium falciparum candidate vaccine based on a six-antigen polyprotein encoded by recombinant poxviruses. Proc Natl Acad Sci U S A. 2004;101:290–5. doi: 10.1073/pnas.0307158101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.John CC, Moormann AM, Sumba PO, Ofulla AV, Pregibon DC, Kazura JW. Gamma interferon responses to Plasmodium falciparum liver-stage antigen 1 and thrombospondin-related adhesive protein and their relationship to age, transmission intensity, and protection against malaria. Infect Immun. 2004;72:5135–42. doi: 10.1128/IAI.72.9.5135-5142.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chougnet C, Lepers JP, Astagneau P, Rason MD, Savel J, Deloron P. Lymphoproliferative responses to synthetic peptides from merozoite ring-infected erythrocyte surface antigen and circumsporozoite protein: a longitudinal study during a falciparum malaria episode. Am J Trop Med Hyg. 1991;45:560–6. doi: 10.4269/ajtmh.1991.45.560. [DOI] [PubMed] [Google Scholar]
- 17.Fidock DA, Gras-Masse H, Lepers JP, et al. Plasmodium falciparum liver stage antigen-1 is well conserved and contains potent B and T cell determinants. J Immunol. 1994;153:190–204. [PubMed] [Google Scholar]
- 18.Gysin J, Barnwell J, Schlesinger DH, Nussenzweig V, Nussenzweig RS. Neutralization of the infectivity of sporozoites of Plasmodium knowlesi by antibodies to a synthetic peptide. J Exp Med. 1984;160:935–40. doi: 10.1084/jem.160.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollingdale MR, Nardin EH, Tharavanij S, Schwartz AL, Nussenzweig RS. Inhibition of entry of Plasmodium falciparum and P. vivax sporozoites into cultured cells; an in vitro assay of protective antibodies. J Immunol. 1984;132:909–13. [PubMed] [Google Scholar]
- 20.Charoenvit Y, Fallarme V, Rogers WO, et al. Development of two monoclonal antibodies against Plasmodium falciparum sporozoite surface protein 2 and mapping of B-cell epitopes. Infect Immun. 1997;65:3430–7. doi: 10.1128/iai.65.8.3430-3437.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller HM, Reckmann I, Hollingdale MR, Bujard H, Robson KJ, Crisanti A. Thrombospondin related anonymous protein (TRAP) of Plasmodium falciparum binds specifically to sulfated glycoconjugates and to HepG2 hepatoma cells suggesting a role for this molecule in sporozoite invasion of hepatocytes. Embo J. 1993;12:2881–9. doi: 10.1002/j.1460-2075.1993.tb05950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schofield L, Ferreira A, Altszuler R, Nussenzweig V, Nussenzweig RS. Interferon-gamma inhibits the intrahepatocytic development of malaria parasites in vitro. J Immunol. 1987;139:2020–5. [PubMed] [Google Scholar]
- 23.Charoenvit Y, Majam VF, Corradin G, et al. CD4+ T-cell– and gamma interferon–dependent protection against murine malaria by immunization with linear synthetic peptides from a Plasmodium yoelii 17-kilodalton hepatocyte erythrocyte protein. Infect Immun. 1999;67:5604–14. doi: 10.1128/iai.67.11.5604-5614.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sedegah M, Hedstrom R, Hobart P, Hoffman S. Protection against malaria by immunization with plasmid DNA encoding circumsporozoite protein. Proc Natl Acad Sci U S A. 1994;91:9866–70. doi: 10.1073/pnas.91.21.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frevert U, Sinnis P, Cerami C, Shreffler W, Takacs B, Nussenzweig V. Malaria circumsporozoite protein binds to heparan sulfate proteoglycans associated with the surface membrane of hepatocytes. J Exp Med. 1993;177:1287–98. doi: 10.1084/jem.177.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sultan AA, Thathy V, Frevert U, et al. TRAP is necessary for gliding motility and infectivity of plasmodium sporozoites. Cell. 1997;90:511–22. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- 27.Kurtis JD, Hollingdale MR, Luty AJ, Lanar DE, Krzych U, Duffy PE. Pre-erythrocytic immunity to Plasmodium falciparum: the case for an LSA-1 vaccine. Trends Parasitol. 2001;17:219–23. doi: 10.1016/s0169-4758(00)01862-7. [DOI] [PubMed] [Google Scholar]
- 28.Jafarshad A, Dziegiel MH, Lundquist R, Nielsen LK, Singh S, Druilhe PL. A novel antibody-dependent cellular cytotoxicity mechanism involved in defense against malaria requires costimulation of monocytes FcgammaRII and FcgammaRIII. J Immunol. 2007;178:3099–106. doi: 10.4049/jimmunol.178.5.3099. [DOI] [PubMed] [Google Scholar]
- 29.Lundquist R, Nielsen LK, Jafarshad A, et al. Human recombinant antibodies against Plasmodium falciparum merozoite surface protein 3 cloned from peripheral blood leukocytes of individuals with immunity to malaria demonstrate antiparasitic properties. Infect Immun. 2006;74:3222–31. doi: 10.1128/IAI.00928-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Migot-Nabias F, Deloron P, Ringwald P, et al. Immune response to Plasmodium falciparum liver stage antigen-1: geographical variations within Central Africa and their relationship with protection from clinical malaria. Trans R Soc Trop Med Hyg. 2000;94:557–62. doi: 10.1016/s0035-9203(00)90086-5. [DOI] [PubMed] [Google Scholar]
- 31.John CC, Zickafoose JS, Sumba PO, King CL, Kazura JW. Antibodies to the Plasmodium falciparum antigens circumsporozoite protein, thrombospondin-related adhesive protein, and liver-stage antigen 1 vary by ages of subjects and by season in a highland area of Kenya. Infect Immun. 2003;71:4320–5. doi: 10.1128/IAI.71.8.4320-4325.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitua AY, Urassa H, Wechsler M, et al. Antibodies against Plasmodium falciparum vaccine candidates in infants in an area of intense and perennial transmission: relationships with clinical malaria and with entomological inoculation rates. Parasite Immunol. 1999;21:307–17. doi: 10.1046/j.1365-3024.1999.00230.x. [DOI] [PubMed] [Google Scholar]
- 33.Wongsrichanalai C, Webster HK, Permpanich B, Chuanak N, Ketrangsri S. Naturally acquired circumsporozoite antibodies and their role in protection in endemic falciparum and vivax malaria. Am J Trop Med Hyg. 1991;44:201–4. doi: 10.4269/ajtmh.1991.44.201. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Z, Xiao L, Branch OH, Kariuki S, Nahlen BL, Lal AA. Antibody responses to repetitive epitopes of the circumsporozoite protein, liver stage antigen-1, and merozoite surface protein-2 in infants residing in a Plasmodium falciparum–hyperendemic area of western Kenya. XIII. Asembo Bay Cohort Project. Am J Trop Med Hyg. 2002;66:7–12. doi: 10.4269/ajtmh.2002.66.7. [DOI] [PubMed] [Google Scholar]
- 35.Alonso PL, Sacarlal J, Aponte JJ, et al. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet. 2005;366:2012–8. doi: 10.1016/S0140-6736(05)67669-6. [DOI] [PubMed] [Google Scholar]
- 36.Alonso PL, Sacarlal J, Aponte JJ, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–20. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 37.Ofulla AV, Moormann AM, Embury PE, Kazura JW, Sumba PO, John CC. Age-related differences in the detection of Plasmodium falciparum infection by PCR and microscopy, in an area of Kenya with holoendemic malaria. Ann Trop Med Parasitol. 2005;99:431–5. doi: 10.1179/136485905X36316. [DOI] [PubMed] [Google Scholar]


