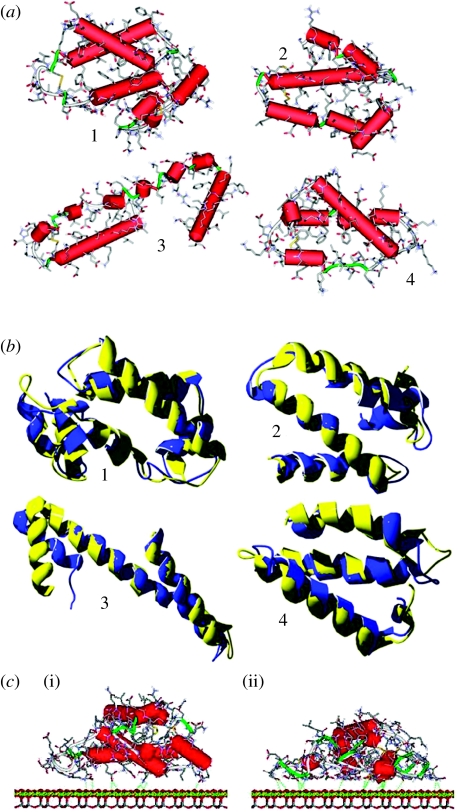
Figure 2.
(a) Starting three-dimensional structures of the individual four subdomains. Hydrogen atoms have been omitted for clarity. Cylinders indicate α-helices (red), while ribbons denote random strands and regular turns (green). (b) Comparison between the starting secondary structures and the minimized ones for the four simulated subdomains, showing the little differences between the optimized (blue ribbon) and the experimental (yellow ribbon) geometries of the backbone. (c) Geometries of the (i) starting and (ii) initial adsorption states of the zone 1 on the chrysotile surface in the dielectric medium: the amino acids close to the surface locally optimize their interactions with the chrysotile planes by partially loosing their secondary α-helical structure. The retained helicoidal features suffer large distortions from their typical conformation, so that H-bonds between adjacent turns are absent. Consequently, these helicoidal strands cannot be classified as genuine α-helices. The detailed chemical structure is shown, omitting the hydrogen atoms for clarity.