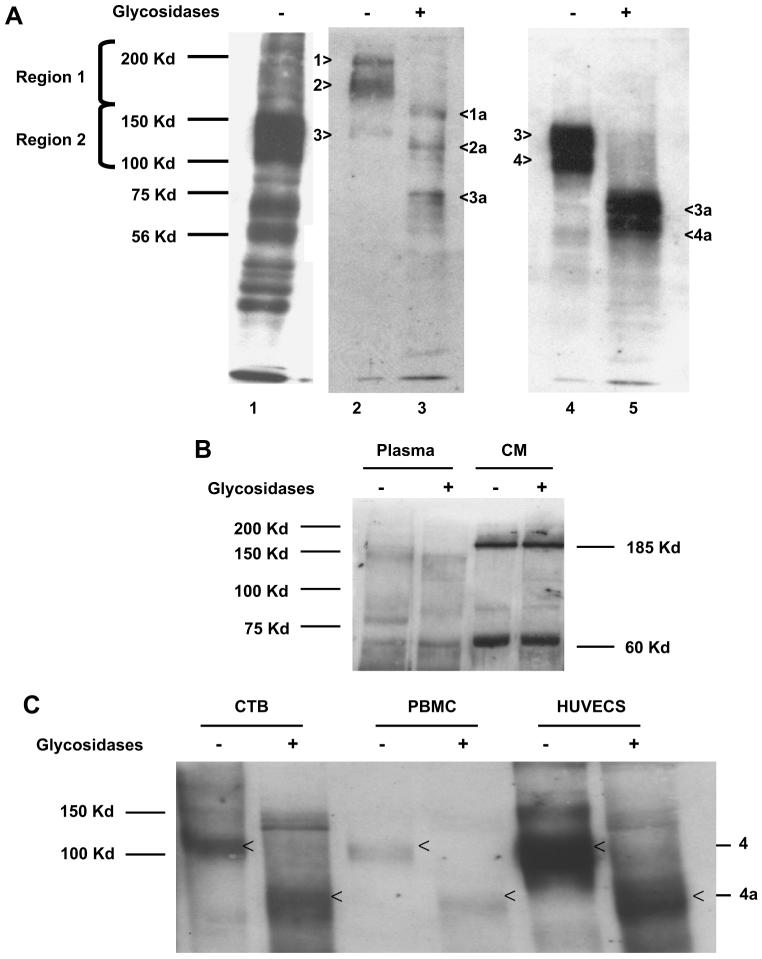
Fig. 4.
Deglycosylation yields sFlt-1 polypeptide variants of differing molecular weights in both villous explant conditioned media and maternal plasma. (A) Western analysis for sFlt-1 was performed using antibody from Sigma, Inc. (St. Louis, MO) after heparin-agarose enrichment of pooled preeclamptic plasma (A, Lane 1); Lanes 2–4 show the proteins eluted from regions 1 and 2, and Lanes 3 and 5 are deglycosylated sFlt-1 variants, respectively. The bands are numbered 1–4 and the deglycosylated forms are denoted by 1a–4a, respectively. (B) Pooled plasma from preeclamptic woman and pooled preeclamptic villous explant hypoxic conditioned media were treated as noted above but with a different antibody from Santa Cruz Biotechnology. The representative Western blot demonstrates unglycosylated forms of sFlt-1, specifically the 150 kDa band in plasma and 185 and 60 kDa bands in conditioned medium. (C) Conditioned medium from cytotrophoblasts (CTB), peripheral blood mononuclear cells (PBMCs) and human uterine microvascular endothelial cells (HUtMVECs) treated with glycosidases also demonstrated that the 100 kDa sFlt-1 is the major form (Band 4 in Lane 4 of Fig. 4A) and when deglycosylated it gives rise to a polypeptide with a molecular weight of 60–70 kDa (Band 4a in lane 5 of 4A) using the Sigma antibody.