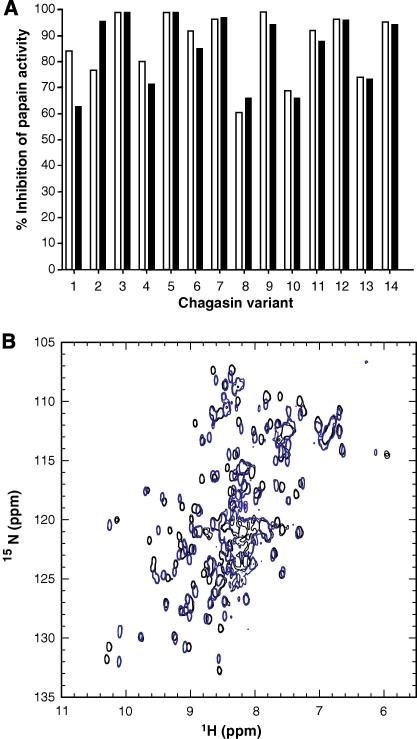
Fig. 2.
Thermo-stability properties of chagasin mutants. (A) Chagasin variants were submitted to high temperatures and subsequently tested for inhibitory activity to evaluate if the mutations introduced caused alterations in their folding state. The inhibitory activity of variants kept on ice was measured in parallel as a control. Chagasin variants were kept on ice (clear bars) or incubated at 70 °C for 20 min (dark bars). The samples were subsequently incubated with papain for 5 min and the residual activity was measured by addition of Z-Phe-Arg-MCA. The experiment was performed twice independently and the variation was below 10%. The graph represents one experiment. The percentage of inhibition was calculated considering the activity of untreated papain as 100%. 1, T32Y; 2, T32S; 3, Y89F; 4, T31V; 5, T31S; 6, T32E; 7, wild type; 8, Y89S; 9, T32A; 10, T31Y; 11, T32V; 12, W93A; 13, P30A; 14, T31A/T32A. (B) NMR spectra of the chagasin variant ΔT31–T32. Comparison of 15N HSQC spectra recorded at 600 MHz (1H) and 298 K, of wild type chagasin (black) with variant ΔT31–T32 (blue), indicating that this variant is properly folded.