Abstract
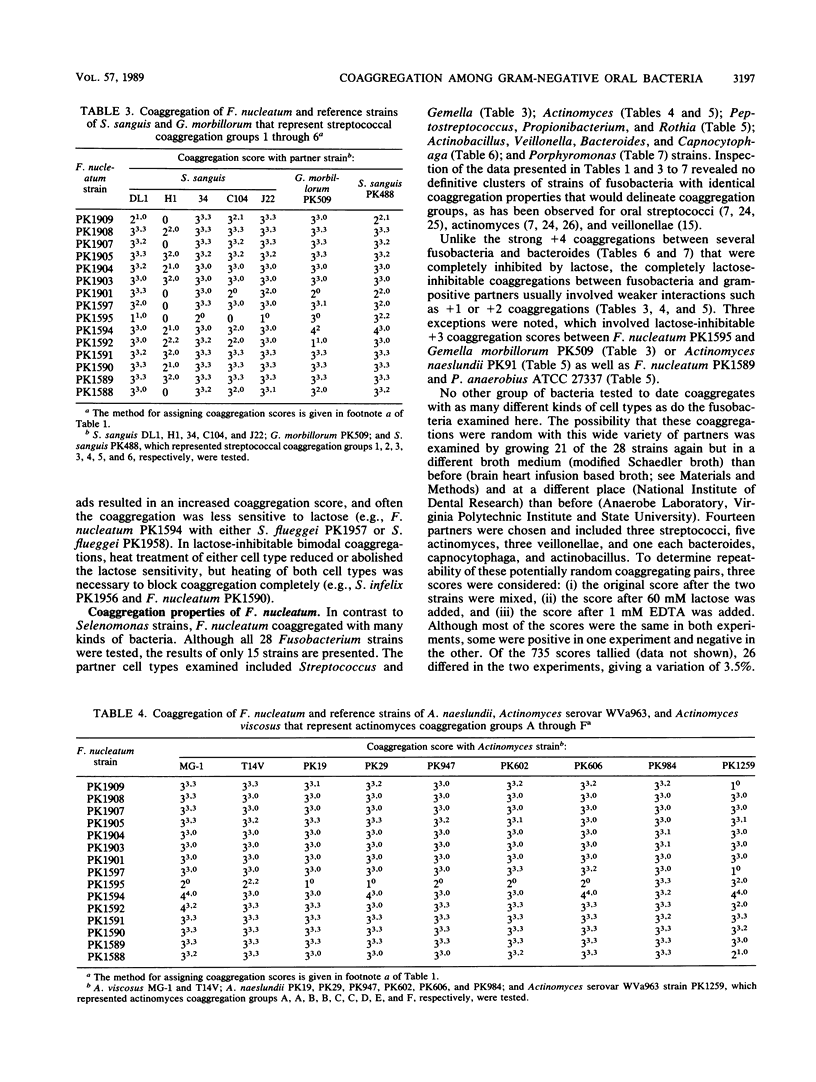
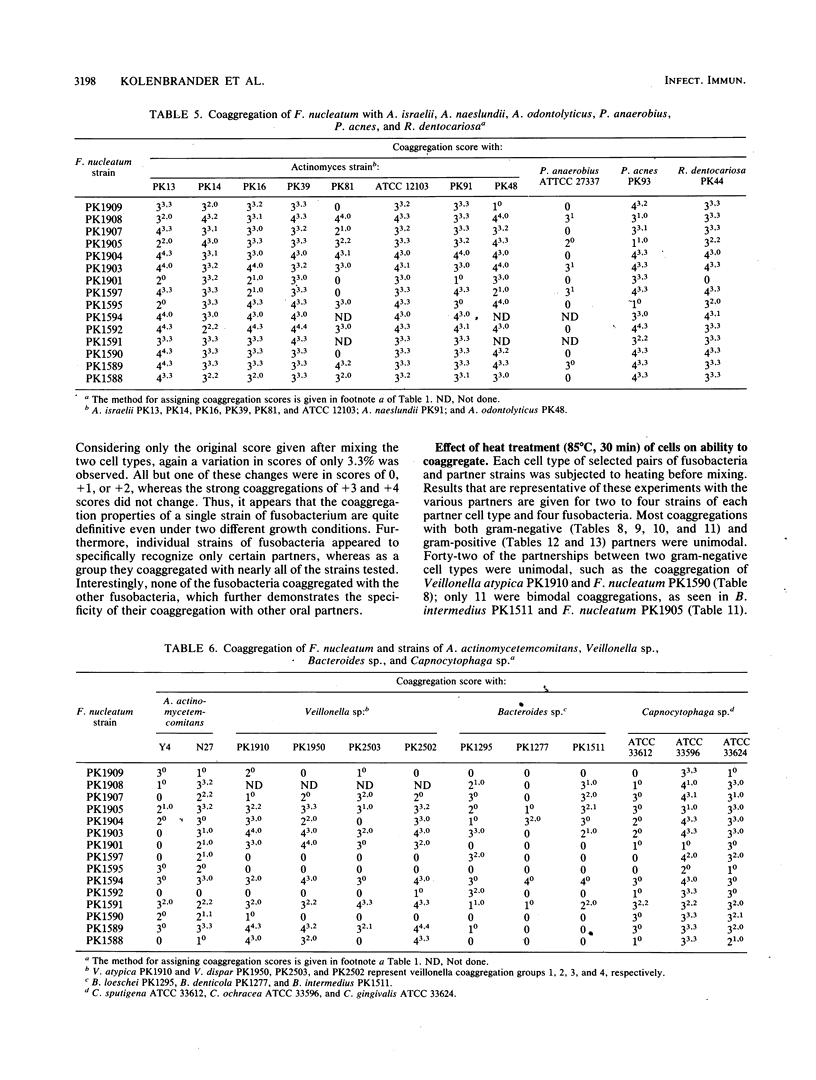
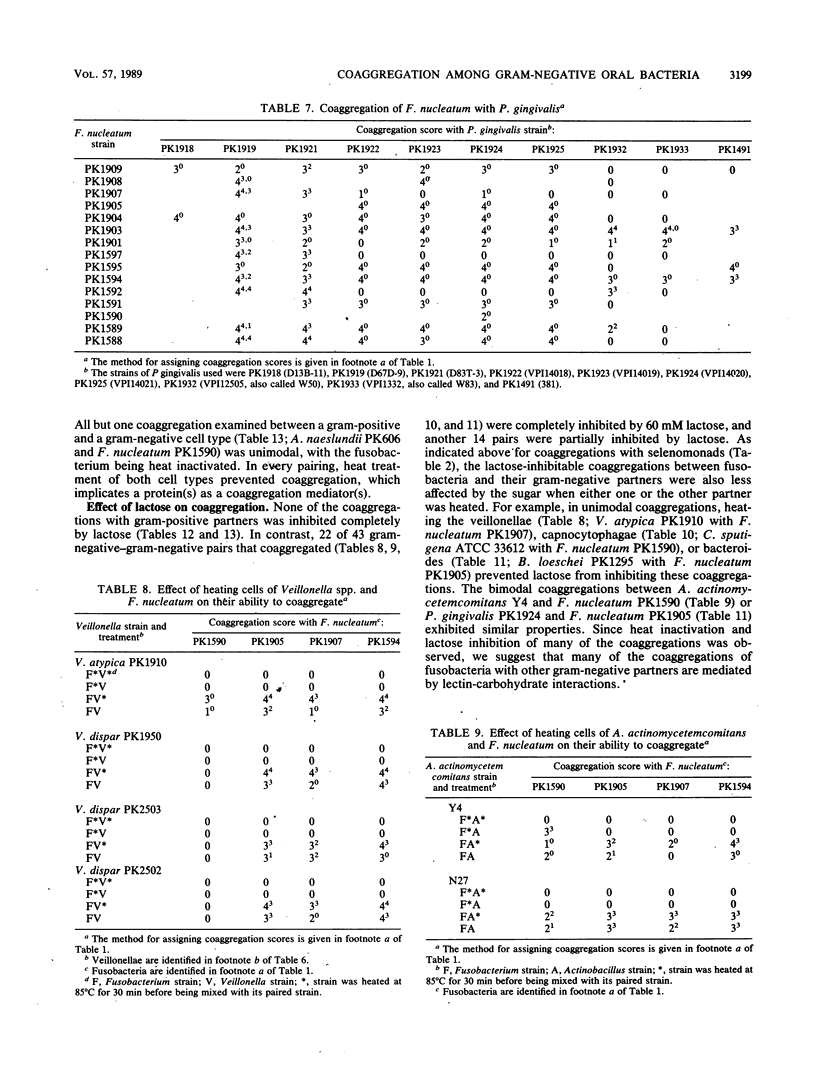
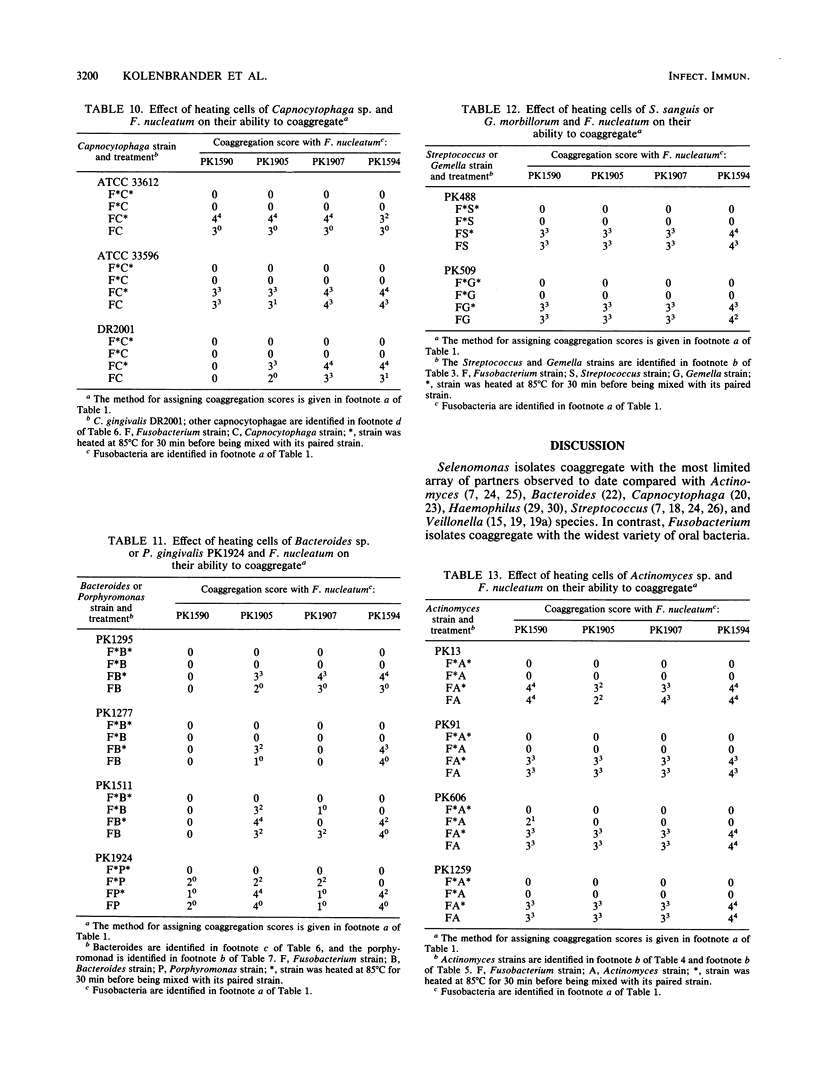
Twenty-eight strains of Fusobacterium nucleatum and 41 Selenomonas strains, including S. sputigena (24 strains), S. flueggei (10 strains), S. infelix (5 strains), and S. noxia (2 strains), were tested for their ability to coaggregate with each other and with 49 other strains of oral bacteria representing Actinobacillus, Actinomyces, Bacteroides, Capnocytophaga, Gemella, Peptostreptococcus, Porphyromonas, Propionibacterium, Rothia, Streptococcus, and Veillonella species. Selenomonads coaggregated with fusobacteria and with Actinomyces naeslundii PK984 but not with any of the other bacteria, including other selenomonads. In contrast, fusobacteria coaggregated with members of all genera, although not with all strains of each species tested. Each fusobacterium strain appeared to have its own set of partners and coaggregation properties, unlike their partners, whose coaggregation properties in earlier surveys delineated distinct coaggregation groups. Coaggregations of fusobacteria with the 63 gram-negative strains were usually inhibited by EDTA, whereas those with the 27 gram-positive strains were usually not inhibited. Likewise, lactose-inhibitable coaggregations were common among some strains of fusobacteria and some strains from each of the genera containing gram-negative partners but were rarely observed with gram-positive partners. Heating the fusobacteria at 85 degrees C for 30 min completely prevented coaggregation with most partners, suggesting the involvement of a protein on the fusobacteria. Heat treatment of many of the gram-negative partners not only enhanced their coaggregation with the fusobacteria but also changed lactose-sensitive coaggregations to lactose-insensitive coaggregations. Although fusobacteria coaggregated with a broader variety of oral partner strains than any other group of oral bacteria tested to date, each fusobacterium exhibited coaggregation with only a certain set of partner strains, and none of the fusobacteria adhered to other strains of fusobacteria, indicating that recognition of partner cell surfaces is selective. The strains of F. nucleatum are heterogeneous and cannot be clustered into distinct coaggregation groups. Collectively, these results indicate that coaggregation between fusobacteria and many gram-negative partners is significantly different from their coaggregation with gram-positive partners. The contrasting variety of partners for fusobacteria and selenomonads supports the concept of coaggregation partner specificity that has been observed with every genus of oral bacteria so far examined.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calhoon D. A., Mayberry W. R., Slots J. Cellular fatty acid and soluble protein profiles of oral fusobacteria. J Dent Res. 1983 Dec;62(12):1181–1185. doi: 10.1177/00220345830620120101. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Herrmann B. F., Höfling J. F., Sundqvist G. K. Degradation of the human proteinase inhibitors alpha-1-antitrypsin and alpha-2-macroglobulin by Bacteroides gingivalis. Infect Immun. 1984 Feb;43(2):644–648. doi: 10.1128/iai.43.2.644-648.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Höfling J. F., Sundqvist G. K. Degradation of albumin, haemopexin, haptoglobin and transferrin, by black-pigmented Bacteroides species. J Med Microbiol. 1984 Aug;18(1):39–46. doi: 10.1099/00222615-18-1-39. [DOI] [PubMed] [Google Scholar]
- Celesk R. A., London J. Attachment of oral Cytophaga species to hydroxyapatite-containing surfaces. Infect Immun. 1980 Aug;29(2):768–777. doi: 10.1128/iai.29.2.768-777.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRienzo J. M., Rosan B. Isolation of a major cell envelope protein from Fusobacterium nucleatum. Infect Immun. 1984 May;44(2):386–393. doi: 10.1128/iai.44.2.386-393.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzink J. L., Socransky S. S., Haffajee A. D. The predominant cultivable microbiota of active and inactive lesions of destructive periodontal diseases. J Clin Periodontol. 1988 May;15(5):316–323. doi: 10.1111/j.1600-051x.1988.tb01590.x. [DOI] [PubMed] [Google Scholar]
- Falkler W. A., Jr, Burger B. W. Microbial surface interactions: reduction of the haemagglutination activity of the oral bacterium Fusobacterium nucleatum by absorption with Streptococcus and Bacteroides. Arch Oral Biol. 1981;26(12):1015–1025. doi: 10.1016/0003-9969(81)90112-6. [DOI] [PubMed] [Google Scholar]
- Falkler W. A., Jr, Hawley C. E. Hemagglutinating activity of Fusobacterium nucleatum. Infect Immun. 1977 Jan;15(1):230–238. doi: 10.1128/iai.15.1.230-238.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D., McBride B. C. Isolation of a membrane-associated Bacteroides gingivalis glycylprolyl protease. Infect Immun. 1987 Dec;55(12):3131–3136. doi: 10.1128/iai.55.12.3131-3136.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. V., Kolenbrander P. E., Andersen R. N., Moore L. V. Coaggregation properties of human oral Veillonella spp.: relationship to colonization site and oral ecology. Appl Environ Microbiol. 1988 Aug;54(8):1957–1963. doi: 10.1128/aem.54.8.1957-1963.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. J. A special relationship between spherical and filamentous microorganisms in mature human dental plaque. Arch Oral Biol. 1972 Mar;17(3):613–616. doi: 10.1016/0003-9969(72)90081-7. [DOI] [PubMed] [Google Scholar]
- Kaufman J., DiRienzo J. M. Isolation of a corncob (coaggregation) receptor polypeptide from Fusobacterium nucleatum. Infect Immun. 1989 Feb;57(2):331–337. doi: 10.1128/iai.57.2.331-337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelstrup J., Funder-Nielsen T. D. Aggregation of oral streptococci with Fusobacterium and Actinomyces. J Biol Buccale. 1974 Dec;2(4):347–362. [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N. Cell to cell interactions of Capnocytophaga and Bacteroides species with other oral bacteria and their potential role in development of plaque. J Periodontal Res. 1984 Nov;19(6):564–569. doi: 10.1111/j.1600-0765.1984.tb01315.x. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N., Holdeman L. V. Coaggregation of oral Bacteroides species with other bacteria: central role in coaggregation bridges and competitions. Infect Immun. 1985 Jun;48(3):741–746. doi: 10.1128/iai.48.3.741-746.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N. Inhibition of coaggregation between Fusobacterium nucleatum and Porphyromonas (Bacteroides) gingivalis by lactose and related sugars. Infect Immun. 1989 Oct;57(10):3204–3209. doi: 10.1128/iai.57.10.3204-3209.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Celesk R. A. Coaggregation of human oral Cytophaga species and Actinomyces israelii. Infect Immun. 1983 Jun;40(3):1178–1185. doi: 10.1128/iai.40.3.1178-1185.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Inouye Y., Holdeman L. V. New Actinomyces and Streptococcus coaggregation groups among human oral isolates from the same site. Infect Immun. 1983 Aug;41(2):501–506. doi: 10.1128/iai.41.2.501-506.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E. Intergeneric coaggregation among human oral bacteria and ecology of dental plaque. Annu Rev Microbiol. 1988;42:627–656. doi: 10.1146/annurev.mi.42.100188.003211. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E. Surface recognition among oral bacteria: multigeneric coaggregations and their mediators. Crit Rev Microbiol. 1989;17(2):137–159. doi: 10.3109/10408418909105746. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Lactose-reversible coaggregation between oral actinomycetes and Streptococcus sanguis. Infect Immun. 1981 Jul;33(1):95–102. doi: 10.1128/iai.33.1.95-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Prevalence of viridans streptococci exhibiting lactose-inhibitable coaggregation with oral actinomycetes. Infect Immun. 1983 Aug;41(2):449–452. doi: 10.1128/iai.41.2.449-452.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOE H., THEILADE E., JENSEN S. B. EXPERIMENTAL GINGIVITIS IN MAN. J Periodontol. 1965 May-Jun;36:177–187. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- Lancy P., Jr, Appelbaum B., Holt S. C., Rosan B. Quantitative in vitro assay for "corncob" formation. Infect Immun. 1980 Aug;29(2):663–670. doi: 10.1128/iai.29.2.663-670.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancy P., Jr, Dirienzo J. M., Appelbaum B., Rosan B., Holt S. C. Corncob formation between Fusobacterium nucleatum and Streptococcus sanguis. Infect Immun. 1983 Apr;40(1):303–309. doi: 10.1128/iai.40.1.303-309.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Bloomquist C. G., Coulter M. C., Fenner L. J., Skopek R. J., Schachtele C. F. Utilization of a continuous streptococcal surface to measure interbacterial adherence in vitro and in vivo. J Dent Res. 1988 Dec;67(12):1455–1460. doi: 10.1177/00220345880670120301. [DOI] [PubMed] [Google Scholar]
- Listgarten M. A., Mayo H., Amsterdam M. Ultrastructure of the attachment device between coccal and filamentous microorganisms in "corn cob" formations of dental plaque. Arch Oral Biol. 1973 May;18(5):651–656. doi: 10.1016/0003-9969(73)90105-2. [DOI] [PubMed] [Google Scholar]
- Mongiello J. R., Falkler W. A., Jr Sugar inhibition of oral Fusobacterium nucleatum haemagglutination and cell binding. Arch Oral Biol. 1979;24(7):539–545. doi: 10.1016/0003-9969(79)90133-x. [DOI] [PubMed] [Google Scholar]
- Moore L. V., Moore W. E., Cato E. P., Smibert R. M., Burmeister J. A., Best A. M., Ranney R. R. Bacteriology of human gingivitis. J Dent Res. 1987 May;66(5):989–995. doi: 10.1177/00220345870660052401. [DOI] [PubMed] [Google Scholar]
- Moore W. E., Hash D. E., Holdeman L. V., Cato E. P. Polyacrylamide slab gel electrophoresis of soluble proteins for studies of bacterial floras. Appl Environ Microbiol. 1980 Apr;39(4):900–907. doi: 10.1128/aem.39.4.900-907.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Cato E. P., Smibert R. M., Burmeister J. A., Palcanis K. G., Ranney R. R. Comparative bacteriology of juvenile periodontitis. Infect Immun. 1985 May;48(2):507–519. doi: 10.1128/iai.48.2.507-519.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Good I. J., Burmeister J. A., Palcanis K. G., Ranney R. R. Bacteriology of experimental gingivitis in young adult humans. Infect Immun. 1982 Nov;38(2):651–667. doi: 10.1128/iai.38.2.651-667.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Hash D. E., Burmeister J. A., Ranney R. R. Bacteriology of severe periodontitis in young adult humans. Infect Immun. 1982 Dec;38(3):1137–1148. doi: 10.1128/iai.38.3.1137-1148.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. A., Kern D. G., Winkler J. R. Identification of a galactose-binding lectin on Fusobacterium nucleatum FN-2. Infect Immun. 1988 May;56(5):1314–1319. doi: 10.1128/iai.56.5.1314-1319.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K., Makinen K. K., Loesche W. J. Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infect Immun. 1986 Jul;53(1):213–220. doi: 10.1128/iai.53.1.213-220.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts T. V., Holdeman L. V., Slots J. Relationships among the oral fusobacteria assessed by DNA-DNA hybridization. J Dent Res. 1983 Jun;62(6):702–705. doi: 10.1177/00220345830620060101. [DOI] [PubMed] [Google Scholar]
- Robrish S. A., Oliver C., Thompson J. Amino acid-dependent transport of sugars by Fusobacterium nucleatum ATCC 10953. J Bacteriol. 1987 Sep;169(9):3891–3897. doi: 10.1128/jb.169.9.3891-3897.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist G., Carlsson J., Herrmann B., Tärnvik A. Degradation of human immunoglobulins G and M and complement factors C3 and C5 by black-pigmented Bacteroides. J Med Microbiol. 1985 Feb;19(1):85–94. doi: 10.1099/00222615-19-1-85. [DOI] [PubMed] [Google Scholar]
- Theilade E., Wright W. H., Jensen S. B., Löe H. Experimental gingivitis in man. II. A longitudinal clinical and bacteriological investigation. J Periodontal Res. 1966;1:1–13. doi: 10.1111/j.1600-0765.1966.tb01842.x. [DOI] [PubMed] [Google Scholar]
- Uitto V. J., Grenier D., Chan E. C., McBride B. C. Isolation of a chymotrypsinlike enzyme from Treponema denticola. Infect Immun. 1988 Oct;56(10):2717–2722. doi: 10.1128/iai.56.10.2717-2722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



