Abstract
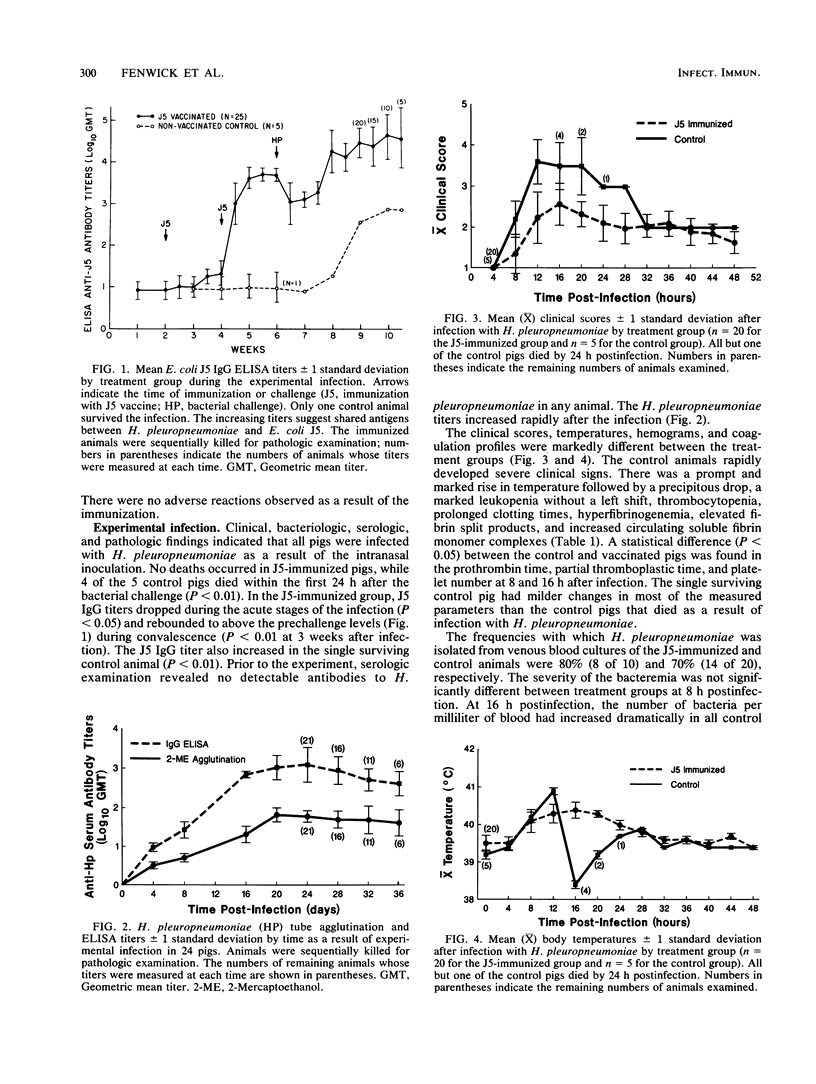
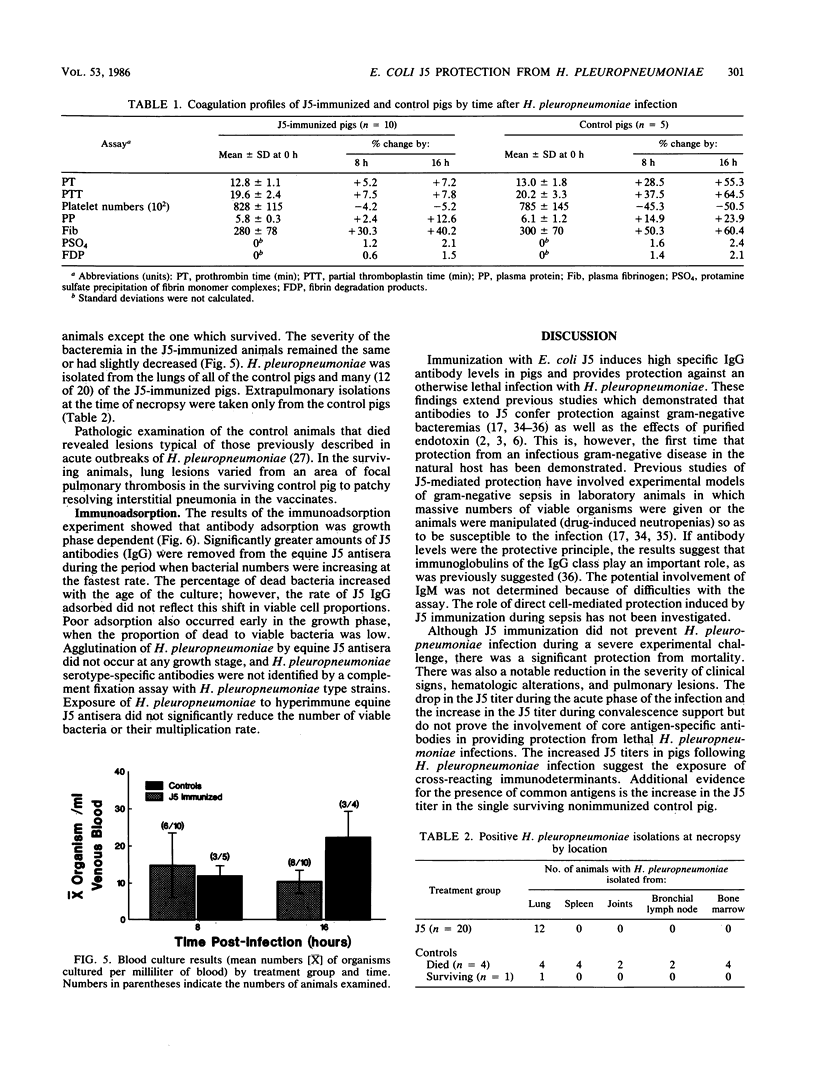
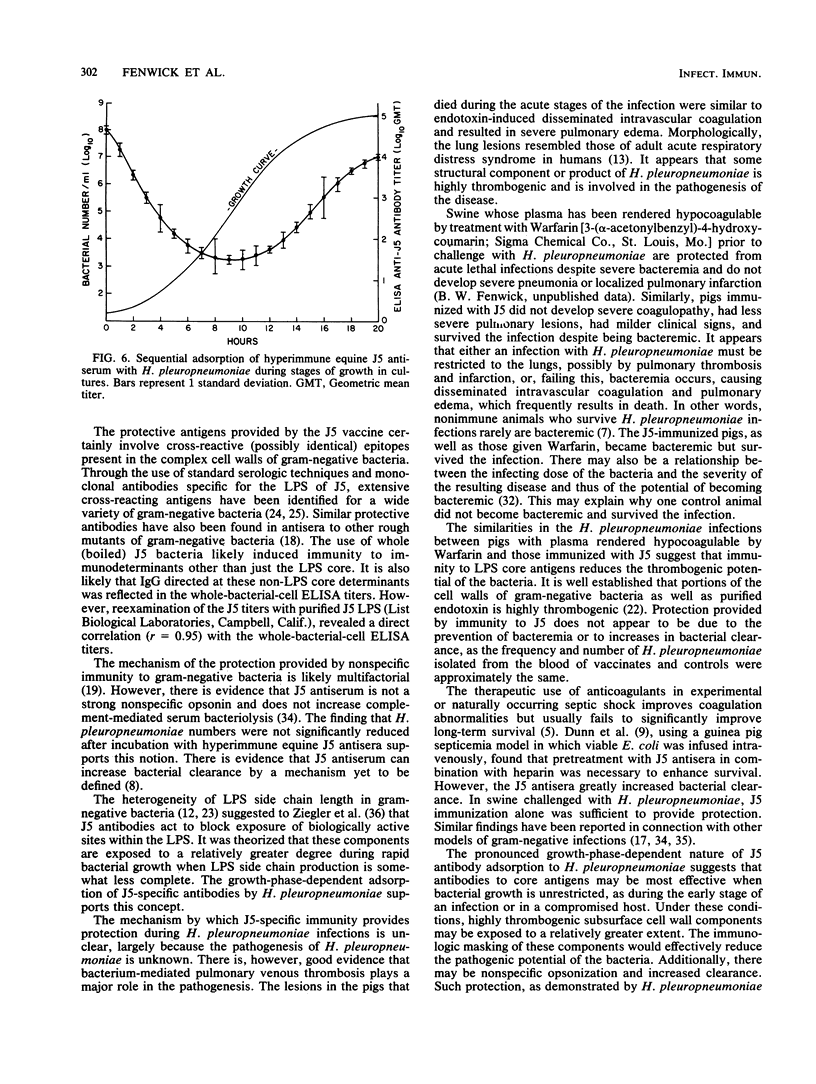
In an investigation of the potential protective effects of immunity against common lipopolysaccharide core antigens of gram-negative bacteria during a severe gram-negative infection in the natural host, we induced Haemophilus pleuropneumoniae infections in weanling pigs immunized with a vaccine of an Rc mutant of Escherichia coli (strain J5). To help define the mechanism involved in J5-mediated protection, we compared the clinical, hematologic, bacteriologic, and serologic responses following an H. pleuropneumoniae infection in J5-immunized pigs with those following an H. pleuropneumoniae infection in nonimmunized control animals. As a result of an intranasal inoculation, all of the control animals and the J5-immunized animals were infected with H. pleuropneumoniae. However, while 80% (4 of 5) of the nonimmunized pigs died within 24 h as a result of the infection, no deaths occurred in the J5-immunized animals. In the immunized group, J5 titers dropped during the acute stages of the infection and rebounded to well above the prechallenge levels during convalescence. The J5 titer also increased in the single surviving control animal. These findings suggest that antibodies against common subsurface components of gram-negative bacterial cell walls correlate with protection from an otherwise lethal challenge of H. pleuropneumoniae but do not prevent infection. Important growth-phase-dependent antigenic changes have been recognized to occur during the growth of H. pleuropneumoniae in cultures (R. Nielson, Nord. Veterinaermed. 28:337-348, 1976). In a study of these changes and during an inquiry into the mechanism of J5 antibody-mediated protection, measured quantities of H. pleuropneumoniae were removed from a broth culture at hourly intervals and used to absorb hyperimmune equine J5 antiserum. Significantly greater amounts of J5-specific antibodies were absorbed during the log phase of bacterial growth than during the early or late phase. The availability of epitopes recognized by J5 antibodies appears to be closely related to the rate of bacterial multiplication. The results of these experiments suggest a mechanism of protection provided by increased immunity to E. coli J5 during gram-negative infections.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendixen P. H., Shewen P. E., Rosendal S., Wilkie B. N. Toxicity of Haemophilus pleuropneumoniae for porcine lung macrophages, peripheral blood monocytes, and testicular cells. Infect Immun. 1981 Sep;33(3):673–676. doi: 10.1128/iai.33.3.673-676.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braude A. I., Douglas H., Davis C. E. Treatment and prevention of intravascular coagulation with antiserum to endotoxin. J Infect Dis. 1973 Jul;128(Suppl):157–164. doi: 10.1093/infdis/128.supplement_1.s157. [DOI] [PubMed] [Google Scholar]
- Braude A. I., Douglas H. Passive immunization against the local Shwartzman reaction. J Immunol. 1972 Feb;108(2):505–512. [PubMed] [Google Scholar]
- Chedid L., Parant M., Parant F., Boyer F. A proposed mechanism for natural immunity to enterobacterial pathogens. J Immunol. 1968 Feb;100(2):292–306. [PubMed] [Google Scholar]
- Corrigan J. J., Jr, Jordan C. M. Heparin therapy in septicemia with disseminated intravascular coagulation. N Engl J Med. 1970 Oct 8;283(15):778–782. doi: 10.1056/NEJM197010082831502. [DOI] [PubMed] [Google Scholar]
- Davis C. E., Ziegler E. J., Arnold K. F. Neutralization of meningococcal endotoxin by antibody to core glycolipid. J Exp Med. 1978 Apr 1;147(4):1007–1017. doi: 10.1084/jem.147.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier P. J., Perino L., Urbance J. Porcine Haemophilus pleuropneumonia: microbiologic and pathologic findings. J Am Vet Med Assoc. 1984 Mar 15;184(6):716–719. [PubMed] [Google Scholar]
- Dunn D. L., Ferguson R. M. Immunotherapy of gram-negative bacterial sepsis: enhanced survival in a guinea pig model by use of rabbit antiserum to Escherichia coli J5. Surgery. 1982 Aug;92(2):212–219. [PubMed] [Google Scholar]
- Dunn D. L., Mach P. A., Cerra F. B., Ferguson R. M. The role of heparin in guinea pig gram negative bacterial sepsis. J Surg Res. 1983 May;34(5):479–485. doi: 10.1016/0022-4804(83)90099-9. [DOI] [PubMed] [Google Scholar]
- ELBEIN A. D., HEATH E. C. THE BIOSYNTHESIS OF CELL WALL LIPOPOLYSACCHARIDE IN ESCHERICHIA COLI. I. THE BIOCHEMICAL PROPERTIES OF A URIDINE DIPHOSPHATE GALACTOSE 4-EPIMERASELESS MUTANT. J Biol Chem. 1965 May;240:1919–1925. [PubMed] [Google Scholar]
- Fulford K. W., Johnson N., Loveday C., Storey J., Tedder R. S. Changes in intra-vascular complement and anti-treponemal antibody titres preceding the Jarisch-Herxheimer reaction in secondary syphilis. Clin Exp Immunol. 1976 Jun;24(3):483–491. [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Häni H., König H., Nicolet J., Scholl E. Zur Haemophilus-Pleuropneumonie beim Schwein. V. Pathomorphologie. Schweiz Arch Tierheilkd. 1973 May;115(5):191–203. [PubMed] [Google Scholar]
- Ito J. I., Jr, Wunderlich A. C., Lyons J., Davis C. E., Guiney D. G., Braude A. I. Role of magnesium in the enzyme-linked immunosorbent assay for lipopolysaccharides of rough Escherichia coli strain J5 and Neisseria gonorrhoeae. J Infect Dis. 1980 Oct;142(4):532–537. doi: 10.1093/infdis/142.4.532. [DOI] [PubMed] [Google Scholar]
- Kidder W. R., Logan L. J., Rapaport S. I., Patch M. J. The plasma protamine paracoagulation test: clinical and laboratory evaluation. Am J Clin Pathol. 1972 Dec;58(6):675–686. doi: 10.1093/ajcp/58.6.675. [DOI] [PubMed] [Google Scholar]
- Liddell F. D. Evaluation of survival in challenge experiments. Microbiol Rev. 1978 Mar;42(1):237–249. doi: 10.1128/mr.42.1.237-249.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M. I., Ziegler E. J., Douglas H., Corbeil L. B., Braude A. I. Induction of immunity against lethal Haemophilus influenzae type b infection by Escherichia coli core lipopolysaccharide. J Clin Invest. 1982 Apr;69(4):742–749. doi: 10.1172/JCI110512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe W. R. Immunization with R mutants of S. Minnesota. I. Protection against challenge with heterologous gram-negative bacilli. J Immunol. 1972 Mar;108(3):601–610. [PubMed] [Google Scholar]
- Mittal K. R., Higgins R., Larivière S., Leblanc D. A 2-mercaptoethanol tube agglutination test for diagnosis of Haemophilus pleuropneumoniae infection in pigs. Am J Vet Res. 1984 Apr;45(4):715–719. [PubMed] [Google Scholar]
- Morrison D. C., Cochrane C. G. Direct evidence for Hageman factor (factor XII) activation by bacterial lipopolysaccharides (endotoxins). J Exp Med. 1974 Sep 1;140(3):797–811. doi: 10.1084/jem.140.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Rick P. D. Size heterogeneity of Salmonella typhimurium lipopolysaccharides in outer membranes and culture supernatant membrane fragments. J Bacteriol. 1980 Nov;144(2):630–640. doi: 10.1128/jb.144.2.630-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutharia L. M., Crockford G., Bogard W. C., Jr, Hancock R. E. Monoclonal antibodies specific for Escherichia coli J5 lipopolysaccharide: cross-reaction with other gram-negative bacterial species. Infect Immun. 1984 Sep;45(3):631–636. doi: 10.1128/iai.45.3.631-636.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelles M. J., Niswander C. A. Mouse monoclonal antibodies reactive with J5 lipopolysaccharide exhibit extensive serological cross-reactivity with a variety of gram-negative bacteria. Infect Immun. 1984 Dec;46(3):677–681. doi: 10.1128/iai.46.3.677-681.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROCTOR R. R., RAPAPORT S. I. The partial thromboplastin time with kaolin. A simple screening test for first stage plasma clotting factor deficiencies. Am J Clin Pathol. 1961 Sep;36:212–219. doi: 10.1093/ajcp/36.3.212. [DOI] [PubMed] [Google Scholar]
- Schultz R. A., Young T. F., Ross R. F., Jeske D. R. Prevalence of antibodies to Haemophilus pleuropneumoniae in Iowa swine. Am J Vet Res. 1982 Oct;43(10):1848–1851. [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983 Jun 15;182(12):1331–1337. [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R., Osborne A. D. Dose response relationship of Haemophilus pleuropneumoniae aerosols in pigs. Can J Comp Med. 1983 Jan;47(1):54–56. [PMC free article] [PubMed] [Google Scholar]
- Ziegler E. J., Douglas H., Sherman J. E., Davis C. E., Braude A. I. Treatment of E. coli and klebsiella bacteremia in agranulocytic animals with antiserum to a UDP-gal epimerase-deficient mutant. J Immunol. 1973 Aug;111(2):433–438. [PubMed] [Google Scholar]
- Ziegler E. J., McCutchan J. A., Douglas H., Braude A. I. Prevention of lethal pseudomonas bacteremia with epimerase-deficient E. coli antiserum. Trans Assoc Am Physicians. 1975;88:101–108. [PubMed] [Google Scholar]
- Ziegler E. J., McCutchan J. A., Fierer J., Glauser M. P., Sadoff J. C., Douglas H., Braude A. I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982 Nov 11;307(20):1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]


