Abstract
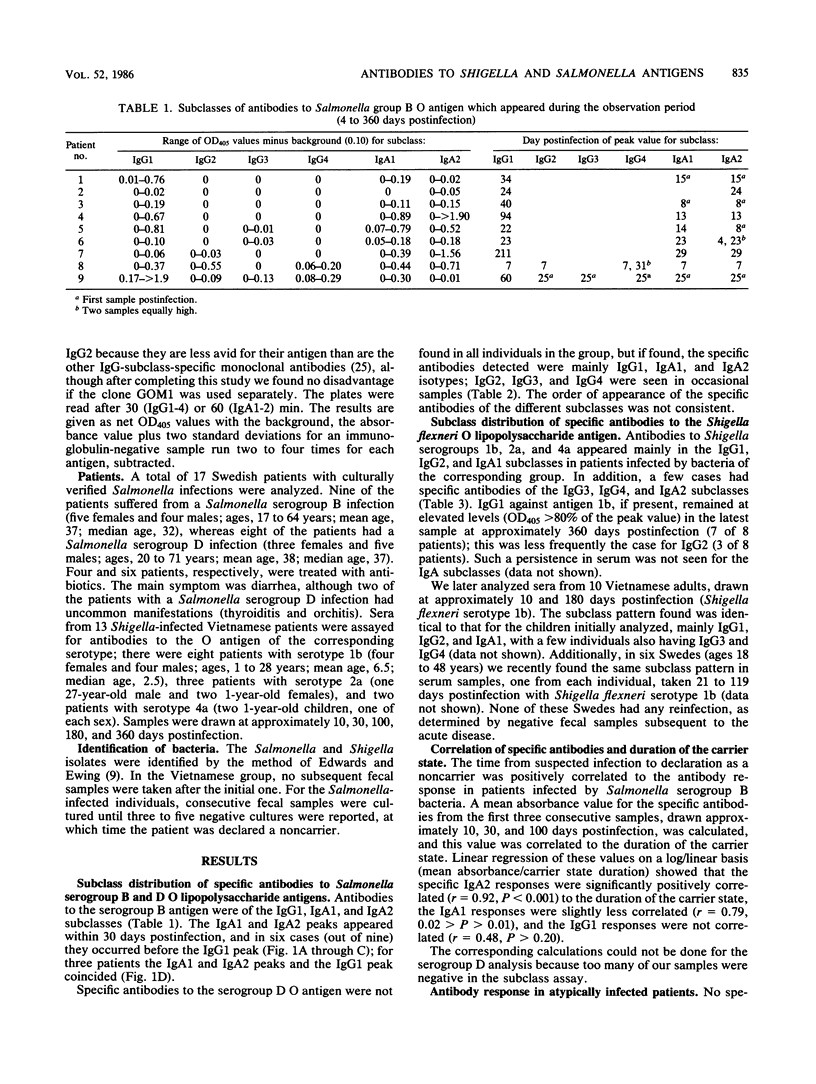
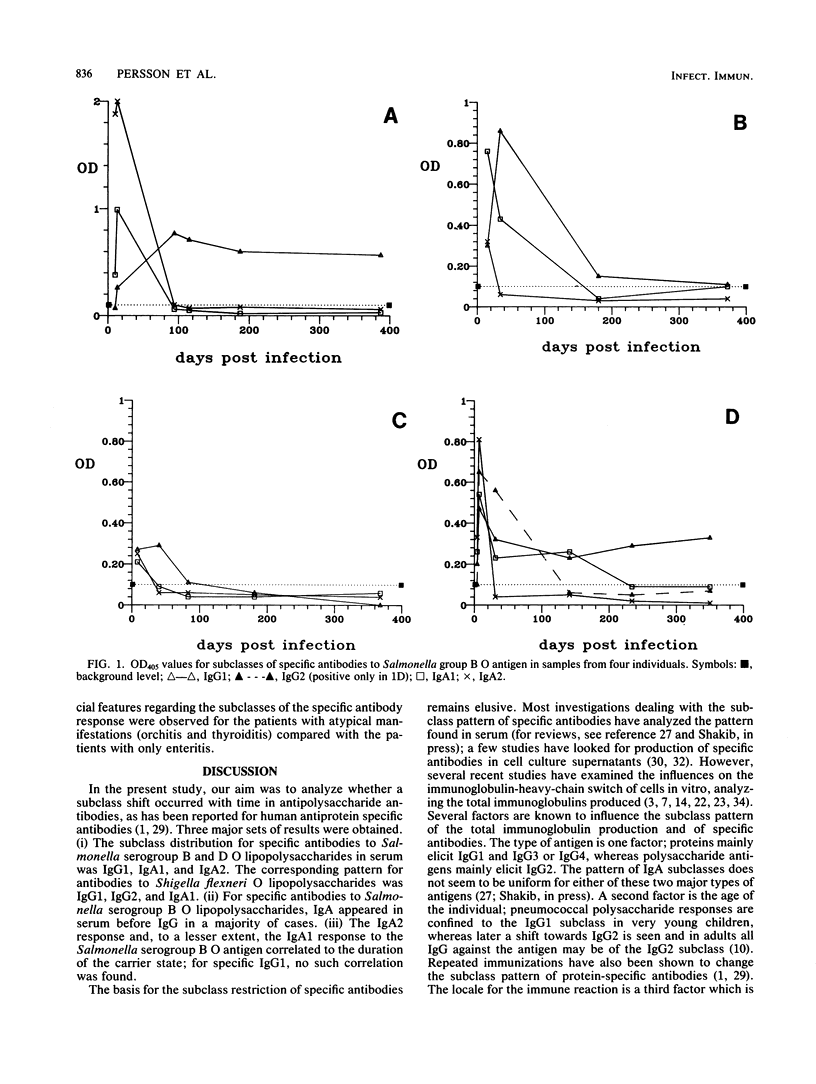
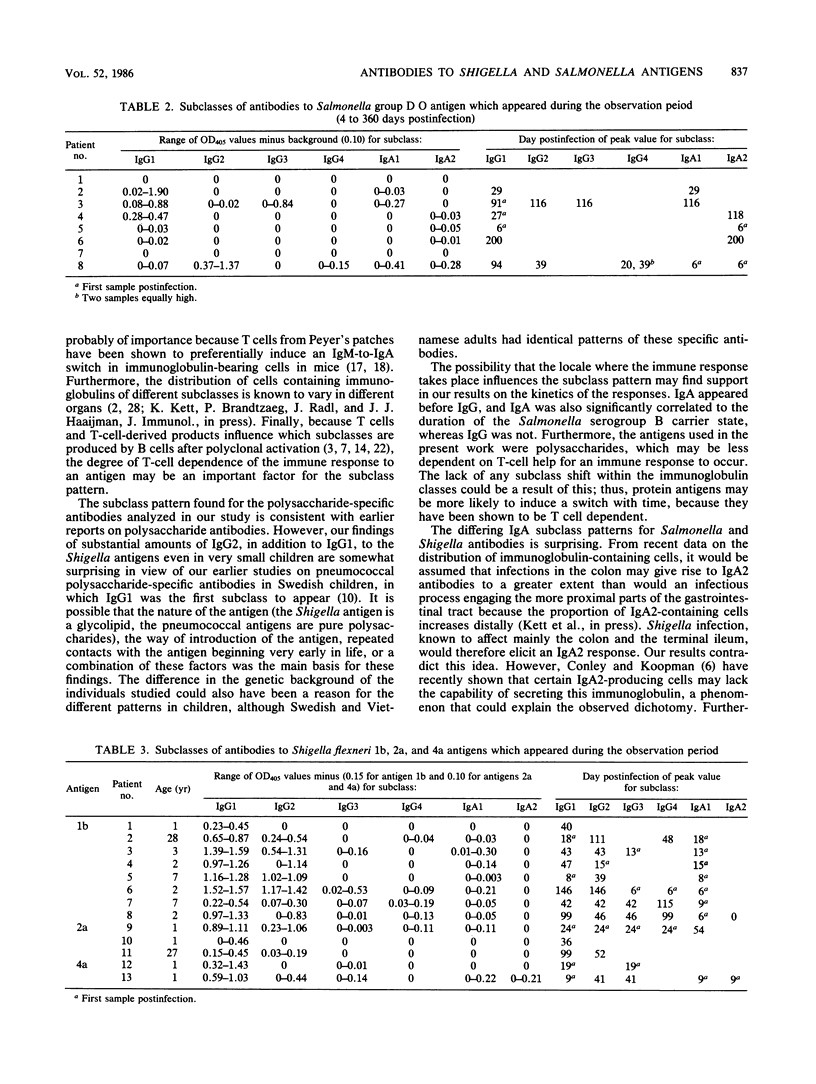
The subclass distribution of human serum antibodies to the O-antigenic lipopolysaccharides of Salmonella serogroups B and D and to Shigella flexneri serotypes 1b, 2a, and 4a lipopolysaccharide antigens were analyzed in an enzyme-linked immunosorbent assay with monoclonal antibodies to the immunoglobulin subclasses. The patients had culture-verified Salmonella (17 Swedes) or Shigella flexneri (23 Vietnamese; 11 children and 12 adults) infections. Consecutive samples drawn during 1 year postinfection were investigated. Antibodies to the Salmonella antigens were mainly of the immunoglobulin G1 (IgG1), IgA1, and IgA2 subclasses. For the Salmonella serogroup B O polysaccharide, the IgA1 and IgA2 subclasses had peak values earlier than (6/9) or coinciding with the IgG1 (3/9) peak value. Furthermore, the IgA2 response to Salmonella serogroup B was positively correlated to the duration of the carrier state (P less than 0.001); the corresponding IgA1 response was less well correlated but was still significant (P less than 0.02). In the case of the Shigella flexneri O polysaccharide, specific antibodies appeared mainly in the IgG1 and IgA1 subclasses. Some IgG2 was also found, surprisingly even in very young patients. No subclass shift with time within the immunoglobulin classes was noted in any of the groups.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalberse R. C., van der Gaag R., van Leeuwen J. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J Immunol. 1983 Feb;130(2):722–726. [PubMed] [Google Scholar]
- André C., André F., Fargier C. Distribution of IgA 1 and IgA 2 plasma cells in various normal human tissues and in the jejunum of plasma IgA-deficient patients. Clin Exp Immunol. 1978 Aug;33(2):327–331. [PMC free article] [PubMed] [Google Scholar]
- Bergstedt-Lindqvist S., Sideras P., MacDonald H. R., Severinson E. Regulation of Ig class secretion by soluble products of certain T-cell lines. Immunol Rev. 1984 Apr;78:25–50. doi: 10.1111/j.1600-065x.1984.tb00475.x. [DOI] [PubMed] [Google Scholar]
- Conley M. E., Kearney J. F., Lawton A. R., 3rd, Cooper M. D. Differentiation of human B cells expressing the IgA subclasses as demonstrated by monoclonal hybridoma antibodies. J Immunol. 1980 Nov;125(5):2311–2316. [PubMed] [Google Scholar]
- Conley M. E., Koopman W. J. In vitro regulation of IgA subclass synthesis. I. Discordance between plasma cell production and antibody secretion. J Exp Med. 1982 Dec 1;156(6):1615–1621. doi: 10.1084/jem.156.6.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A., Pettersson S., Ruuth E., Forni L. Immunoglobulin C gene expression. IV. Alternative control of IgG1-producing cells by helper cell-derived B cell-specific growth or maturation factors. Eur J Immunol. 1983 Mar;13(3):269–272. doi: 10.1002/eji.1830130319. [DOI] [PubMed] [Google Scholar]
- DRAY S. Three gamma-globulins in normal human serum revealed by monkey precipitins. Science. 1960 Nov 4;132(3436):1313–1314. doi: 10.1126/science.132.3436.1313. [DOI] [PubMed] [Google Scholar]
- Freijd A., Hammarström L., Persson M. A., Smith C. I. Plasma anti-pneumococcal antibody activity of the IgG class and subclasses in otitis prone children. Clin Exp Immunol. 1984 May;56(2):233–238. [PMC free article] [PubMed] [Google Scholar]
- GREY H. M., KUNKEL H. G. H CHAIN SUBGROUPS OF MYELOMA PROTEINS AND NORMAL 7S GAMMA-GLOBULIN. J Exp Med. 1964 Aug 1;120:253–266. doi: 10.1084/jem.120.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick R. B., Greisman S. E., Woodward T. E., DuPont H. L., Dawkins A. T., Snyder M. J. Typhoid fever: pathogenesis and immunologic control. 2. N Engl J Med. 1970 Oct 1;283(14):739–746. doi: 10.1056/NEJM197010012831406. [DOI] [PubMed] [Google Scholar]
- Isakson P. C., Puré E., Vitetta E. S., Krammer P. H. T cell-derived B cell differentiation factor(s). Effect on the isotype switch of murine B cells. J Exp Med. 1982 Mar 1;155(3):734–748. doi: 10.1084/jem.155.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. B., Köhl S., Bessler W. G. Polyclonal activation of B-lymphocytes in vivo by Salmonella typhimurium lipoprotein. Infect Immun. 1983 Mar;39(3):1481–1484. doi: 10.1128/iai.39.3.1481-1484.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K., Carlsson H. E., Neringer R., Lindberg A. A. Application and usefulness of enzyme immunoassay for diagnosis of Salmonella typhimurium infection. Scand J Infect Dis. 1980;12(1):41–47. doi: 10.3109/inf.1980.12.issue-1.08. [DOI] [PubMed] [Google Scholar]
- Kawanishi H., Saltzman L. E., Strober W. Mechanisms regulating IgA class-specific immunoglobulin production in murine gut-associated lymphoid tissues. I. T cells derived from Peyer's patches that switch sIgM B cells to sIgA B cells in vitro. J Exp Med. 1983 Feb 1;157(2):433–450. doi: 10.1084/jem.157.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanishi H., Saltzman L., Strober W. Mechanisms regulating IgA class-specific immunoglobulin production in murine gut-associated lymphoid tissues. II. Terminal differentiation of postswitch sIgA-bearing Peyer's patch B cells. J Exp Med. 1983 Sep 1;158(3):649–669. doi: 10.1084/jem.158.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M. Shigella and Salmonella diarrhoeal disease. Trop Doct. 1979 Jan;9(1):4–9. doi: 10.1177/004947557900900103. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A., Holme T. Evaluation of some extraction methods for the preparation of bacterial lipopolysaccharides for structural analysis. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(5):751–759. doi: 10.1111/j.1699-0463.1972.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Lowe J., Bird P., Hardie D., Jefferis R., Ling N. R. Monoclonal antibodies (McAbs) to determinants on human gamma chains: properties of antibodies showing subclass restriction or subclass specificity. Immunology. 1982 Oct;47(2):329–336. [PMC free article] [PubMed] [Google Scholar]
- Martinez-Alonso C., Coutinho A., Augustin A. A. Immunoglobulin C-gene expression. I. The commitment to IgG subclass of secretory cells is determined by the quality of the nonspecific stimuli. Eur J Immunol. 1980 Sep;10(9):698–702. doi: 10.1002/eji.1830100908. [DOI] [PubMed] [Google Scholar]
- Mayumi M., Kuritani T., Kubagawa H., Cooper M. D. IgG subclass expression by human B lymphocytes and plasma cells: B lymphocytes precommitted to IgG subclass can be preferentially induced by polyclonal mitogens with T cell help. J Immunol. 1983 Feb;130(2):671–677. [PubMed] [Google Scholar]
- Persson M. A., Hammarström L., Smith C. I. Enzyme-linked immunosorbent assay for subclass distribution of human IgG and IgA antigen-specific antibodies. J Immunol Methods. 1985 Apr 8;78(1):109–121. doi: 10.1016/0022-1759(85)90334-5. [DOI] [PubMed] [Google Scholar]
- Shakib F., Stanworth D. R. Human IgG subclasses in health and disease. (A review). Part I. Ric Clin Lab. 1980 Jul-Sep;10(3):463–479. doi: 10.1007/BF02938793. [DOI] [PubMed] [Google Scholar]
- Shakib F., Stanworth D. R. Human IgG subclasses in health and disease. (A review). Part II. Ric Clin Lab. 1980 Oct-Dec;10(4):561–580. doi: 10.1007/BF02906696. [DOI] [PubMed] [Google Scholar]
- Skvaril F., Morell A. Distribution of IgA subclasses in sera and bone marrow plasma cells of 21 normal individuals. Adv Exp Med Biol. 1974;45(0):433–435. doi: 10.1007/978-1-4613-4550-3_51. [DOI] [PubMed] [Google Scholar]
- Smith C. I., Aarli J. A., Hammarström L., Persson M. A. IgG subclass distribution of myasthenia gravis thymoma-associated antiskeletal muscle antibodies. Neurology. 1984 Aug;34(8):1094–1096. doi: 10.1212/wnl.34.8.1094. [DOI] [PubMed] [Google Scholar]
- Stevens R., Dichek D., Keld B., Heiner D. IgG1 is the predominant subclass of in vivo- and in vitro- produced anti-tetanus toxoid antibodies and also serves as the membrane IgG molecule for delivering inhibitory signals to anti-tetanus toxoid antibody-producing B cells. J Clin Immunol. 1983 Jan;3(1):65–69. doi: 10.1007/BF00919140. [DOI] [PubMed] [Google Scholar]
- TERRY W. D., FAHEY J. L. SUBCLASSES OF HUMAN GAMMA-2-GLOBULIN BASED ON DIFFERENCES IN THE HEAVY POLYPEPTIDE CHAINS. Science. 1964 Oct 16;146(3642):400–401. doi: 10.1126/science.146.3642.400. [DOI] [PubMed] [Google Scholar]
- Thompson P. M., McLachlan S. M., Parkes A., Clark F., Howel D., Rees Smith B. The IgG subclass distribution of thyroglobulin antibody synthesized in culture. Scand J Immunol. 1983 Aug;18(2):123–129. doi: 10.1111/j.1365-3083.1983.tb00848.x. [DOI] [PubMed] [Google Scholar]
- Vaerman J. P., Heremans J. F. Subclasses of human immunoglobulin a based on differences in the alpha polypeptide chains. Science. 1966 Aug 5;153(3736):647–649. doi: 10.1126/science.153.3736.647. [DOI] [PubMed] [Google Scholar]
- Walker L., Johnson G. D., MacLennan I. C. The IgG subclass responses of human lymphocytes to B-cell activators. Immunology. 1983 Oct;50(2):269–272. [PMC free article] [PubMed] [Google Scholar]


