Summary
Background
In 2004, cases of HIV and syphilis were reported in an indigenous community in the Peruvian Amazon. This study sought to determine the prevalence of HIV and syphilis in four remote communities of the same indigenous ethnic group located further from an urban center than the original community, and to identify risk factors for HIV and syphilis transmission.
Methods
Rapid and confirmatory tests for HIV and syphilis were performed. A questionnaire elicited demographic information, risk factors for sexually transmitted infections, and knowledge/beliefs about HIV/AIDS.
Results
We collected 282 blood samples and conducted interviews with 281 (99.6%) participants. The confirmed syphilis prevalence rate was 3.2% (9/282; 3.7% (5/135) for men and 2.7% (4/147) for women). The confirmed HIV prevalence rate was 0.7% (2/282), with both infections in men who had sex with men (MSM). Self-reported MSM activity was 39.7%. There was poor knowledge about HIV infection, transmission, and prevention, and low acceptance of known prevention methods.
Conclusions
HIV and syphilis are now prevalent in remote Amazonian communities of an indigenous group in Peru. Expansion of the HIV epidemic into the Amazon requires an urgent public health response.
Keywords: HIV, Syphilis, Prevalence, Indigenous populations, Homosexual men, Amazon, Peru
Introduction
Indigenous peoples comprise 45% of the 28.7 million inhabitants of Peru (July 2007 estimate),1 yet limited data on sexually transmitted infections (STIs) are available in this population.2–4 Furthermore, while considerable attention is paid to the risks of exposing immigrants to emerging infectious diseases when remote regions like the Amazon Basin are inhabited by non-natives,5 far less attention is paid to the risks among indigenous persons coming into contact with the Western world by traveling into growing cities, as with the spread of HIV in our study. Most of Peru’s indigenous peoples live in the Andes region and are of Quechua or Aymara origin. Only an estimated 300 000 (1.0% of the total population, 2.3% of the indigenous population)6–8 inhabit the Amazon region of the country, even more isolated from mainstream Peruvian society. An estimated 65 ethnic groups, an equal number of languages,7 and varying sexual practices are described.9,10 HIV/AIDS is still considered a primarily urban disease in Peru, and rural indigenous groups in particular are not often targeted in risk reduction efforts. However, the large Amazonian department of Loreto now has the fourth highest rate of HIV infection among the 24 departments in Peru; Loreto also has the highest proportion of Amazonian indigenous persons and is now considered a high-risk zone for HIV transmission.11,12
In 2004, the Department of Epidemiology of the Ministry of Health in Yurimaguas, the capital of Alto Amazonas Province in Loreto, received the first report of a death due to AIDS in an indigenous person in the region.2 Further investigation revealed alarming rates of both HIV and syphilis infection in the patient’s village of origin, a small community located 70 km from Yurimaguas, one hour by canoe and then 12 hours on foot. The prevalence rates of HIV and syphilis in the adults of the community were found to be 7.5% and 6.3%, respectively, much higher than national averages.2 The prevalence of HIV infection among men who reported sex with other men was even higher at 19%.2 The discovery of both HIV and syphilis in a rural indigenous community was disturbing and merited further investigation. In 2006, we began a study of the entire indigenous Amazonian ethnic group to which this community belongs. The study included HIV and syphilis prevalence determination and a qualitative component. In this paper we present a preliminary report of HIV and syphilis prevalence in four communities of this ethnic group, located in remote rural zones in Alto Amazonas Province. If present at all, HIV is presumed to have entered this population only recently and we restricted the study to individuals aged ≥15 years.
Methods
Study population
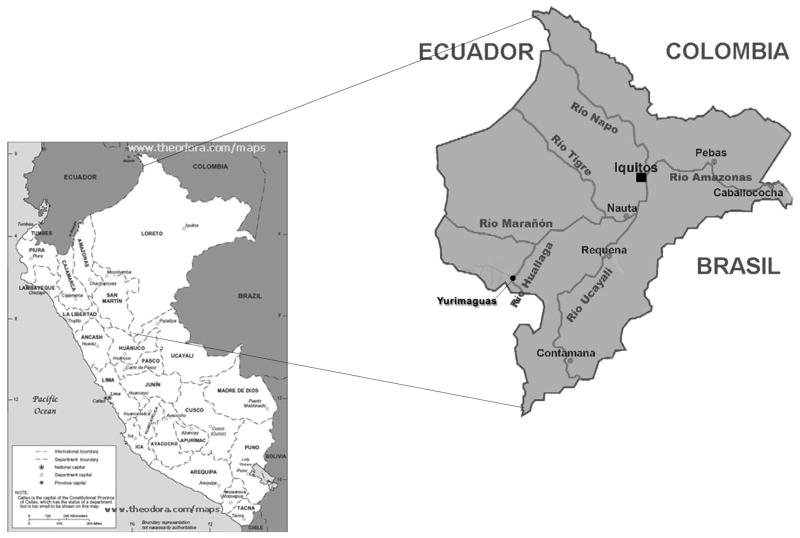
This study was carried out in a district of the Alto Amazonas Province, which is home to an estimated 14 254 members of this indigenous ethnic group, in 72 communities (2006 estimate, CF, unpublished data). The four study communities were located near a remote town within this district, 10–12 hours by motorized canoe or ≥48 hours by foot from Yurimaguas, the nearest semi-urban center in the region (Figure 1). A government-sponsored health center in the town serves a population with 90% of clients coming from this same indigenous ethnic group, a census-based population of 4111 identified individuals in 18 communities (2006 estimate, CF, unpublished data). The four study communities were all located within a one-hour walking distance of the health center.
Figure 1.
The four study communities were located near the city of Yurimaguas in the Amazonian Department of Loreto.
Data collection
Study procedures were explained to all prospective participants with the assistance of an interpreter, in the context of community meetings. Pre-test counseling was also provided at this time. Afterwards, in compliance with local cultural tradition, permission to proceed was sought from the highest authority in each community, a man known as the Apu. Written permission, via signature and/or fingerprint for illiterate persons, was obtained from the head of each family and each individual member of the family who chose to participate. A blood sample was then taken from each willing participant and coded. Rapid HIV and syphilis tests were performed on site using the HIV-1/2 Ag-Ab, Determine™ (Abbott, Japan) test and the rapid plasma reagin (RPR) Nosticon II G2™ (bioMerieux, Netherlands) test, respectively. Serum samples were stored at − 20 °C and transported to the laboratory at the Santa Gema Hospital in Yurimaguas where those samples testing positive for HIV infection by rapid test were confirmed by ELISA. All samples that tested positive for syphilis by RPR were transported to Lima for confirmatory fluorescent treponemal antibody absorption test (FTA ABS) at the Universidad Peruana Cayetano Heredia. Rapid HIV positive samples were also sent to Lima for confirmatory Western blot at Peru’s National Institute of Health. Rapid HIV and RPR test results were presented to patients on site and post-test counseling was provided.
After blood collection, each participant responded to our questionnaire on demographic information, risk factors for STI transmission, and knowledge and beliefs about HIV/AIDS. These were administered by members of the study team with the aid of two interpreters, one male and one female. Results were analyzed using SPSS™ version 13.0 (SPSS, Inc., Chicago, IL, USA) statistical software for possible predictors of infection with HIV and/or syphilis, as well as to further our understanding of the unique characteristics and practices of the study population. Statistical significance was calculated using Chi-square with Yate’s correction or Fisher’s exact test for categorical data and Student’s t-test for continuous data.
Results
We obtained samples from 282 individuals (100% of those approached), representing 55.5% of the total adult population of the four communities; 281 participants (99.6% of persons permitting blood tests) agreed to complete the survey. Other adults lived in the communities, but were unavailable due to absence, usually for work, at the times of our visits. Among the 282 participants, 135 (48%) were men, 147 (52%) were women, and all were ≥ 15 years of age.
Prevalence of HIV and syphilis
Of the 282 participants, six (2.1%) tested positive for HIV by the HIV rapid test. Unexpectedly, only two samples (0.7%) were confirmed positive by ELISA and Western blot. Both confirmed HIV-seropositive individuals were males who reported sex with other males. One of these men also reported a history of working as a sex worker in Iquitos approximately 10 years prior to this study. Eleven of the 282 participants (3.9%) tested positive for syphilis infection by RPR, and nine of these samples were available for confirmation with FTA ABS (two samples hemolyzed). All nine samples were confirmed positive by FTA ABS, providing a confirmed prevalence rate of 3.2%. Syphilis prevalence was therefore between 3.7% (confirmed) and 4.4% (unconfirmed) for men and between 2.7% (confirmed) and 3.4% (unconfirmed) for women. No one was co-infected with HIV and syphilis. The mean age of participants was 31.5 years (range 15–75 years). The mean ages of participants infected with HIV or testing positive for syphilis by RPR were 28.0 years and 46.0 years, respectively.
Risk factors
We identified a number of risk factors associated with the presence of an STI in the study population; 96.3% (130/135) of men and 87.1% (128/147) of women reported being sexually active. Of the sexually active men who answered the question, 39.7% (50/126) reported sex with another man at least once and 13.2% (17/129) reported sex with another man within the past year. Both of the men who tested positive for HIV reported ever having sex with other men, while one reported sex with a man within the past year. None of the women reported sex with other women. Of the men who tested positive for syphilis, 16.7% (1/6) reported sex with a man. In addition, 29.1% (37/127) of sexually active men who answered the question reported having sex with someone other than their partner, either male or female, within the past year. Of the sexually active women, 2.3% (3/128) reported doing so. As noted in previous studies of this indigenous Amazonian ethnic group, polygamy is practiced by some men of higher social or political status.2,13
Only 46.6% of the study population reported knowing what a condom was, and only 19.9% reported ever using a condom. Among sexually active adults, 12.4% (32/258) reported a previous STI, defined as pain on urination, a genital ulcer, swollen inguinal lymph nodes, pus from the penis (men), or foul smelling discharge (women). None of the participants reported intravenous drug use, but 26.0% of the men and 4.8% of the women had at least one tattoo, all of which were non-professional.
The majority of the study population (59.4%) was not aware of the existence of HIV/AIDS at the start of the study, and only 15.7% of those who had knowledge of the disease were aware that condoms could be used to prevent transmission (6.4% of the population). Comparing those who were HIV or RPR positive with those who tested negative, the infected group was less likely to have heard of HIV/AIDS, more likely to report never having used a condom, and more likely to report a previous STI than the uninfected group (Table 1). However, there was no statistically significant association between HIV or syphilis infection and knowledge of HIV/AIDS, condom use, or previous STI.
Table 1.
Comparison of characteristics of study participants with and without HIV or syphilis
| HIV- or RPR-positive (N = 13) | Uninfected (N = 268) | Total respondents (N = 281) | p-Value | |
|---|---|---|---|---|
| No knowledge of HIV/AIDS | 9 (69.2) | 158 (59.0) | 167 (59.4) | 0.7 |
| No knowledge of condoms | 8 (61.5) | 142 (53.0) | 150 (53.4) | 0.7 |
| Condom use, never | 12 (92.3) | 213 (79.5) | 225 (80.1) | 0.5 |
| Previous STIa | 2 (15.4) | 30 (11.2) | 32 (11.4) | 0.6 |
| Tattoo | 2 (15.4) | 40 (14.9) | 42 (14.9) | 1.0 |
| MSM/sexually active males who answered (%) | 3/8 (37.5) | 47/118 (39.8) | 50/126 (39.7) | 1.0 |
| Formal education | ||||
| ≤Primary school | 12 (92.3) | 218 (81.3) | 230 (81.9) | 0.3 |
| >Primary school | 1 (7.7) | 50 (18.7) | 51 (18.1) | |
| Illiterate in Spanish | 8 (61.5) | 154 (57.5) | 162 (57.7) | 1.0 |
| Travel to urban centerb | 12 (92.3) | 229 (85.4) | 241 (85.8) | 0.7 |
| Age in years (± SD) | 43.0 (19.8) | 31.1 (12.5) | 31.6 (13.1) | 0.02 |
RPR, rapid plasma reagin; SD, standard deviation. Results are n (%) unless otherwise stated.
STI = sexually transmitted infection as defined by self-report of history of penile/vaginal discharge, pain with urination, genital ulcer, or enlarged inguinal lymph nodes.
Travel to Yurimaguas at least once.
Discussion
This study confirms the presence of HIV and syphilis in communities of this indigenous Amazonian ethnic group, located more remotely from an urban center (such as Yurimaguas) than the community in which these infections were first discovered in 2004. In addition, it establishes prevalences of HIV and syphilis in these four communities that are equal to and higher than the national averages for HIV and syphilis, respectively. The HIV/AIDS epidemic is no longer confined to the urban centers of Peru, and has in fact penetrated isolated areas of the Amazon region alongside syphilis, and probably other STIs.14,15 While no significant differences were noted besides older age in the HIV or RPR positive persons, many risk behaviors for the continued and potentially rapid spread of HIV were encountered, suggesting an urgent need for educational and STI control interventions in these communities. We (CZ, HR, EG) are currently gathering further qualitative and quantitative data from focus groups and interviews to learn more about knowledge, attitudes, practices, and behaviors from a more representative sample.
In addition to the risk factors identified within the communities themselves, there are a number of other factors that make this population particularly vulnerable to the spread of HIV. Despite the fact that most members of this indigenous Amazonian ethnic group continue to live in small communities isolated from the majority Mestizo and European origin population of Peru, there is substantial migration between the communities and the city of Yurimaguas (85.8% of participants in this study), providing a persistent route by which individuals may become infected with HIV in the urban setting and then reintroduce the virus into their home communities. These trips are generally taken a couple of times a year to sell surplus crops and livestock. Encounters with both male and female sex workers in the city are not uncommon for the men of the communities (community interviews and focus groups by ECB, June 2006, unpublished data). However, in contrast to the social mixing that takes place in Yurimaguas, most members of this indigenous Amazonian ethnic group continue the tradition of forming partnerships and marrying almost exclusively within their own ethnic group.13 This pattern, combined with the high prevalence of men who have sex with men (MSM) within the population, the frequency of extramarital sex, the low knowledge about HIV/AIDS, and low rates of condom use, means that even a small number of new infections in men of this ethnic group could have a substantial impact on the health and well being of a large proportion of the group’s population.
The pattern of HIV infection that is emerging in this indigenous Amazonian ethnic group mirrors the pattern in the broader Peruvian population. Though the national prevalence of HIV infection in Peru is between 0.2% and 0.6%,11,16 the prevalence among MSM averages between 11% and 14%.11,16–18 According to the Ministry of Health’s latest analysis, HIV prevalence among MSM is highest in Lima (22%); Iquitos, the capital of the department of Loreto, has the highest prevalence in the Amazon region (11%).11 Other studies of the MSM population in Peru have found HIV prevalence of 14.5% in Iquitos and 7.5% in Pucallpa, another city in the Amazon region.16 The prevalence of syphilis in Peru was estimated at 0.56% in 2002, with the coastal and Amazon regions being more affected than the Andes region.11
While over half the estimated adult population was sampled, we would have liked to sample more community residents. Many families (both men and women) worked daily in the fields, located far from village centers, and were unavailable to us when we were present. In addition, we learned that some young men were absent from their villages during our study, working in the local mines. While absent persons may be more mobile (e.g., miners with higher risk), they may also have been more gainfully employed and busy (e.g., farmers with lower risk), so we do not know whether our prevalence findings are a bit low or high compared to the true rates of HIV and syphilis infection. Despite these limitations, however, we sampled >95% of the total adult population in two of the four study communities and we were able to obtain survey data from nearly all (99.6%) of the participants tested for HIV and syphilis.
The extent to which HIV/AIDS has already established itself in this indigenous Amazonian ethnic group as a whole, as well as in other indigenous populations of the region, is unknown. HIV testing is not available in the rural clinics that serve these populations. Increasing access to affordable testing for these populations is a priority, as is the need for the implementation of culturally appropriate HIV prevention education and STI control. Individuals of this ethnic group continue to communicate primarily in their native language, though most men who trade in the urban centers speak some Spanish as well. Few women speak Spanish and most possess little decision making power outside of the home.13 This language barrier, along with the low levels of education and high rates of illiteracy in the population, especially among the women (81.6% of women and 31.3% of men in the study population reported being illiterate), must be taken into account when designing such a program. In addition, sexual activity between men in this culture has remained largely stigma-free. Risk reduction education must work within the cultural norms of the society to decrease HIV transmission without introducing prejudice and increasing the potential for discrimination.
Education should focus on improving knowledge and understanding of HIV/AIDS and its modes of transmission, as well as on the proper use of condoms and their role in preventing infection. It is essential that this initiative include efforts to improve societal acceptance of the condom and its use in both heterosexual and homosexual encounters. Due to clearly defined gender roles and social stratification, education should be gender sensitive, with efforts made to empower women to reduce their own risk of infection.18 An interactive educational model, utilizing discussion, role-playing, and possibly theater production would likely increase efficacy while bridging the barriers of illiteracy and the general lack of experience with didactic education. Advocacy of delayed sexual debut in adolescents, reducing the number of sexual partners, and condom use are potentially valuable components.19–21 Finally, collaboration with the established tribal and political leaders of each community, as well as with local health promoters would greatly improve the efficacy and sustainability of any prevention program.
The high rate of false-positive HIV rapid tests was an unexpected finding and remains unexplained. Similar phenomena have been noted in certain populations of Hispanic origin and in regions where malaria is endemic.22,23 There is the possibility of a cross-reactive antigen yet to be discovered in this ethnic group. We were not able to sequence the viral subtypes, but others report circulation of subtypes B and F in the Amazon Basin.24 Serological and molecular typing of the strands of virus found in this population may provide insight into its initial routes of entry into the population, as well as direct future treatment programs.25
Peru recently began a program to provide free antiretroviral treatment to all those in need in 2004.26 However, drug shortages can occur and treatment is sometimes sporadic.26 Since the recent publication of the 2004 data outlining the discovery of HIV and syphilis in a community of this indigenous Amazonian ethnic group, the Pan-American Health Organization (PAHO), along with Peru’s Ministry of Health (MINSA), began sponsorship of a study of HIV and syphilis prevalence and risk behaviors in this population that includes an anthropologic component. The results of this multidisciplinary study are intended to provide a framework for the design of adequate interventions for this population. However, the Amazon is a vast territory and control of HIV and other STIs in the region requires far more epidemiologic data and an urgent public health response.
Acknowledgments
This study was supported in part by the Emphasis Program, the Institute for Global Health, and the Overall Fellowship of Vanderbilt University School of Medicine. It was also supported in part by the Fogarty International Center, NIH, ‘Framework’ grant #R25TW007766. These sponsors did not play any role in the study design, in the collection, analysis or interpretation of data, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
Footnotes
Ethical considerations: This study was approved by the institutional review boards of the Universidad Peruana Cayetano Heredia and the Vanderbilt University. All study participants gave informed consent.
Conflict of interest: No conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The World Factbook. Country profile: Peru. [accessed July 2008]; Available at: https://www.cia.gov/library/publications/the-world-factbook/geos/pe.html.
- 2.Zavaleta C, Fernandez C, Konda K, Valderrama Y, Vermund SH, Gotuzzo E. High prevalence of HIV and syphilis in a remote native community of the Peruvian Amazon. Am J Trop Med Hyg. 2007;76:703–5. [PMC free article] [PubMed] [Google Scholar]
- 3.Medeot S, Nates S, Recalde A, Gallego S, Maturano E, Giordano M, et al. Prevalence of antibody to human T cell lymphotropic virus types 1/2 among aboriginal groups inhabiting northern Argentina and the Amazon region of Peru. Am J Trop Med Hyg. 1999;60:623–9. doi: 10.4269/ajtmh.1999.60.623. [DOI] [PubMed] [Google Scholar]
- 4.Zunt JR, La Rosa AM, Peinado J, Lama JR, Suarez L, Pun M, et al. Risk factors for HTLV-II infection in Peruvian men who have sex with men. Am J Trop Med Hyg. 2006;74:922–5. [PMC free article] [PubMed] [Google Scholar]
- 5.Chavers LS, Vermund SH. An introduction to emerging and reemerging diseases. In: Lashley FR, Durham JD, editors. Emerging infectious diseases: trends and issues. New York: Springer Publishing; 2007. pp. 3–24. [Google Scholar]
- 6.Butler RA. Tropical rainforests: human inhabitants. A place out of time: tropical rainforests and the perils they face. 9 January 2006. [accessed July 2008]; Available at: http://rainforests.mongabay.com/07tsa_indian_estimates.htm.
- 7.Lama A. Peru: indigenous peoples still facing colour gap. Latin American Report. InterPress Third World News Agency (IPS). August 1999. [accessed July 2008]; Available at: www.apc.org.nz/lac/articles/news990820f.htm.
- 8.Peru: country reports on human rights practices—2006. Released by the Bureau of Democracy, Human Rights, and Labor, US Department of State. March 6, 2007. [accessed July 2008]; Available at: http://www.state.gov/g/drl/rls/hrrpt/2006/78902.htm.
- 9.Hern WM. Polygyny and fertility among the Shipibo of the Peruvian Amazon. Pop Stud. 1992;46:53–64. [PubMed] [Google Scholar]
- 10.Mogollón GC, Haver AB. Sexual and reproductive rights and health perceptions, problems, and priorities identified by Asháninka women of Peru’s Río Ene region. International Women’s Health Coalition. [accessed October 14, 2007]; Available at: http://www.iwhc.org/resources/acpcexecsummary.cfm.
- 11.Peru: Ministry of Health; 2006. [accessed July 2008]. Análisis de la situación epidimiológica del VIH/SIDA en el Peru—bases epidemiológicas para la prevención y control. Available at: http://www.oge.sld.pe/publicaciones/pub_asis/asis19.pdf. [Google Scholar]
- 12.Peru: The National Institute of Statistics and Informatics; [accessed July 2008]. Perú: la población de las comunidades indígenas de la Amazonía. Available at: http://www1.inei.gob.pe/biblioineipub/bancopub/Est/Lib0001/Libro.htm. [Google Scholar]
- 13.Fuentes A. Organización social. In: Alba P, editor. Porque las piedras no mueren: historia, sociedad y ritos de los Chayahuita del Alto Amazonas. Lima, Peru: Centro Amazonico de Anthropologia y Aplicacion Practica (CAAAP); 1988. pp. 103–53. [Google Scholar]
- 14.Paris M, Gotuzzo E, Goyzueta G, Aramburu J, Caceres CF, Crawford D, et al. Motorcycle taxi drivers and sexually transmitted infections in a Peruvian Amazon city. Sex Transm Dis. 2001;28:11–3. doi: 10.1097/00007435-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Paris M, Gotuzzo E, Goyzueta G, Aramburu J, Caceres CF, Castellano T, et al. Prevalence of gonococcal and chlamydial infections in commercial sex workers in a Peruvian Amazon city. Sex Transm Dis. 1999;26:103–7. doi: 10.1097/00007435-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 16.UNAIDS update on HIV/AIDS in Peru. [accessed July 2008]; Available at: http://data.unaids.org/Publications/Fact-Sheets01/peru_en.pdf.
- 17.Cáceres CF. HIV among gay and other men who have sex with men in Latin America and the Caribbean: a hidden epidemic? AIDS. 2002;16(Suppl 3):23–33. doi: 10.1097/00002030-200212003-00005. [DOI] [PubMed] [Google Scholar]
- 18.Linn JG, Garnelo L, Husaini BA, Brown C, Benzaken AS, Stringfield YN. HIV prevention for indigenous people of the Amazon Basin. Cell Mol Biol. 2001;47:1009–15. [PubMed] [Google Scholar]
- 19.Trujillo L, Muñoz D, Gotuzzo E, Yi A, Watts DM. Sexual practices and prevalence of HIV, HTLV-I/II, and Treponema pallidum among clandestine female sex workers in Lima, Peru. Sex Transm Dis. 1999;26:115–8. doi: 10.1097/00007435-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Tabet S, Sanchez J, Lama J, Goicochea P, Campos P, Rouillon M, et al. HIV, syphilis and heterosexual bridging among Peruvian men who have sex with men. AIDS. 2002;16:1271–7. doi: 10.1097/00002030-200206140-00010. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez J, Gotuzzo E, Escamilla J, Carrillo C, Moreyra L, Stamm W, et al. Sexually transmitted infections in female sex workers: reduced by condom use but not by a limited periodic examination program. Sex Transm Dis. 1998;25:82–9. doi: 10.1097/00007435-199802000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Zacharias NM, Athanassaki ID, Sangi-Haghpeykar H, Gardner MO. High false-positive rate of human immunodeficiency virus rapid serum screening in a predominantly Hispanic prenatal population. J Perinatol. 2004;24:743–7. doi: 10.1038/sj.jp.7211184. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh K, Javeri KN, Mohanty D, Parmar BD, Surati RR, Joshi SH. False positive serological tests in acute malaria. Br J Biomed Sci. 2001;58:20–3. [PubMed] [Google Scholar]
- 24.Vicente AC, Otsuki K, Silva NB, Castilho MC, Barros FS, Pieniazek D, et al. The HIV epidemic in the Amazon Basin is driven by prototypic and recombinant HIV-1 subtypes B and F. J Acquir Immune Defic Syndr. 2000;23:327–31. doi: 10.1097/00126334-200004010-00008. [DOI] [PubMed] [Google Scholar]
- 25.Machado LF, Vallinoto AC, Souza MI, Azevedo VN, Ishak MO, Ishak R. Serological and molecular typing of HIV type 1 infection in the Tiriyo tribe, a native Indian community of the Amazon region of Brazil. AIDS Res Hum Retroviruses. 2006;22:1267–70. doi: 10.1089/aid.2006.22.1267. [DOI] [PubMed] [Google Scholar]
- 26.Cohen J. HIV/AIDS: Latin America & Caribbean. Peru: universal access: more goal than reality. Science. 2006;313:489. doi: 10.1126/science.313.5786.489. [DOI] [PubMed] [Google Scholar]