Figure 1.
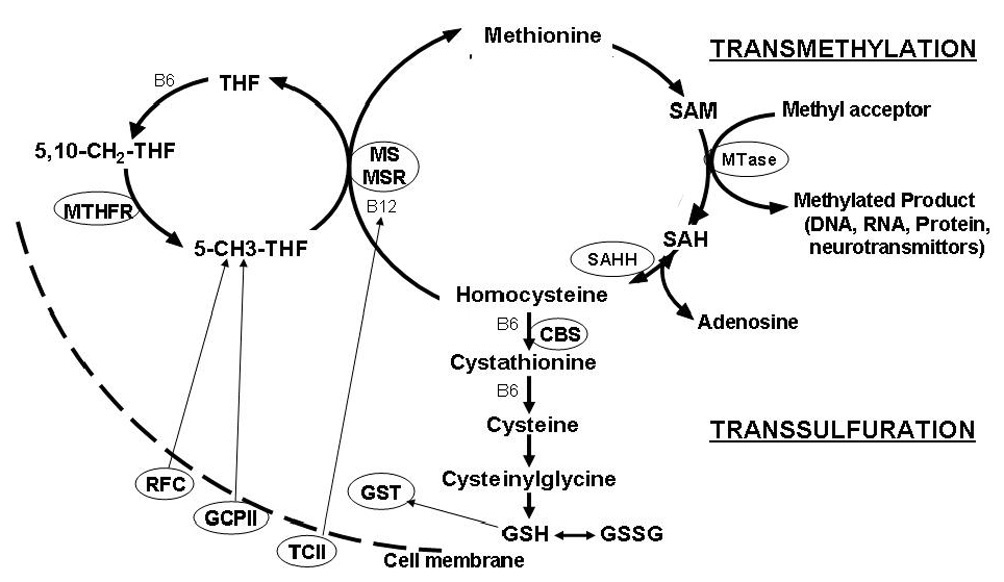
An overview of the pathways involved in folate-dependent methionine transmethylation and transsulfuration. Methylenetetrahydrofolate (MTHFR) catalyzes the synthesis of 5-methyltetrahydrofolate (5-CH3-THF) from 5,10,methylenetetrahydrofolfate (5,10-CH2THF). The methyl group from 5-CH3THF is transferred to homocysteine to regenerate methionine via the folate/B12-dependent methionine synthase (MS) reaction. Methionine synthase reductase (MSR) maintains the B12 cofactor in a reduced state for optimal MS activity. Methionine is then activated to S-adenosylmethionine (SAM), the major methyl donor for multiple cellular methyltransferase (MTase) reactions. After methyl group transfer, SAM is converted to SAH which is further metabolized to homocysteine and adenosine by a reversible reaction catalyzed by SAH hydrolase (SAHH). Homocysteine may be permanently removed from the methionine cycle by irreversible conversion to cystathionine by B6-dependent cystathionine beta synthase (CBS) which initiates the transsulfuration pathway. Cystathionine is subsequently converted to cysteine, the rate limiting amino acid for the synthesis of the tripeptide, glutathione (Glu-Cys-Gly). Reduced active glutathione (GSH) is in dynamic equilibrium with the oxidized disulfide GSSG form of glutathione. Reduced folates are transported from the plasma into the cell by the reduced folate carrier (RFC). Transport of folate into the intestinal mucosa is mediated by glutamate carboxypepsidase II (GCPII). Vitamin B12 is transported into the cell bound to the B12 transport protein transcobalmin II (TCII)
