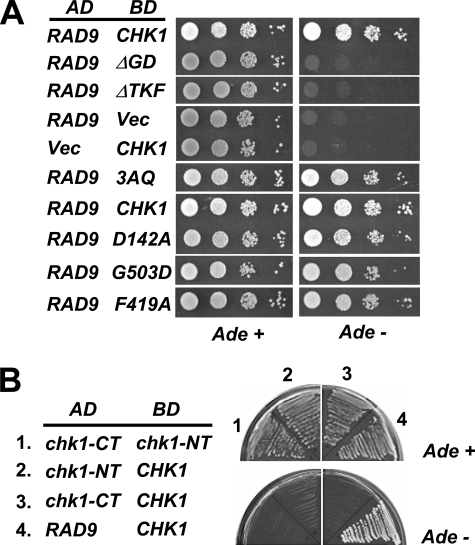
FIGURE 4.
The GD and TRF domains but not the ATRMec1 phosphorylation sites on Chk1 are required for interaction with Rad9p. A, two-hybrid reporter cells were co-transformed with a plasmid expressing Rad9 fused to the GAL4 AD and a plasmid carrying wild-type or mutant Chk1 fused to the GAL4 DNA BD. Transformants were spotted in a series of dilutions on the media indicated. Interaction between AD and BD fusion proteins allowed growth on the media lacking adenine. D142A, catalytically inactive mutant. B, the Chk1 N terminus (chk1-NT, 1–304) and the C terminus (chk1-CT, 281–527) were fused to the GAL4 activation and DNA binding domains to test for interactions between these two domains and between these domains with the full-length protein (CHK1). The transformants were streaked out on media with or without adenine.