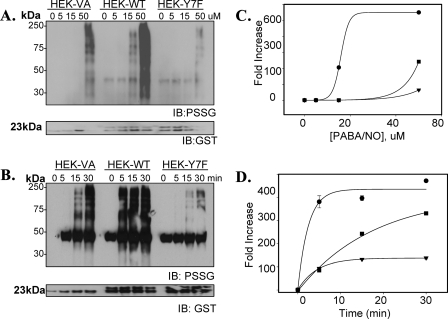
FIGURE 5.
GSTπ enzymatic activity is crucial for protein S-glutathionylation. HEK293 cells were transiently transfected with vector (HEK-VA), wild-type GSTπ (HEK-WT), or an enzymatically inactive mutant form of GSTπ (HEK-Y7F). Concentration (A) and time dependence (B) effects following PABA/NO treatment illustrate that increased ectopic expression of GSTπ stimulates, whereas mutation of the catalytic tyrosine in the enzyme active site diminishes S-glutathionylation (PSSG). The corresponding relative abundance of modified proteins was plotted as the fold increase compared with untreated control for concentration (C) and time (D) dependence. Experimental data were fitted with standard sigmoid, 2-parameter exponential rise to maximum (Sigma Plot 10, SyStat). HEK-WT cells, •; HEK-VA cells, ▪; and HEK-Y7F cells, ▴; n = 3; p < 0.01. IB, immunoblot.