Abstract
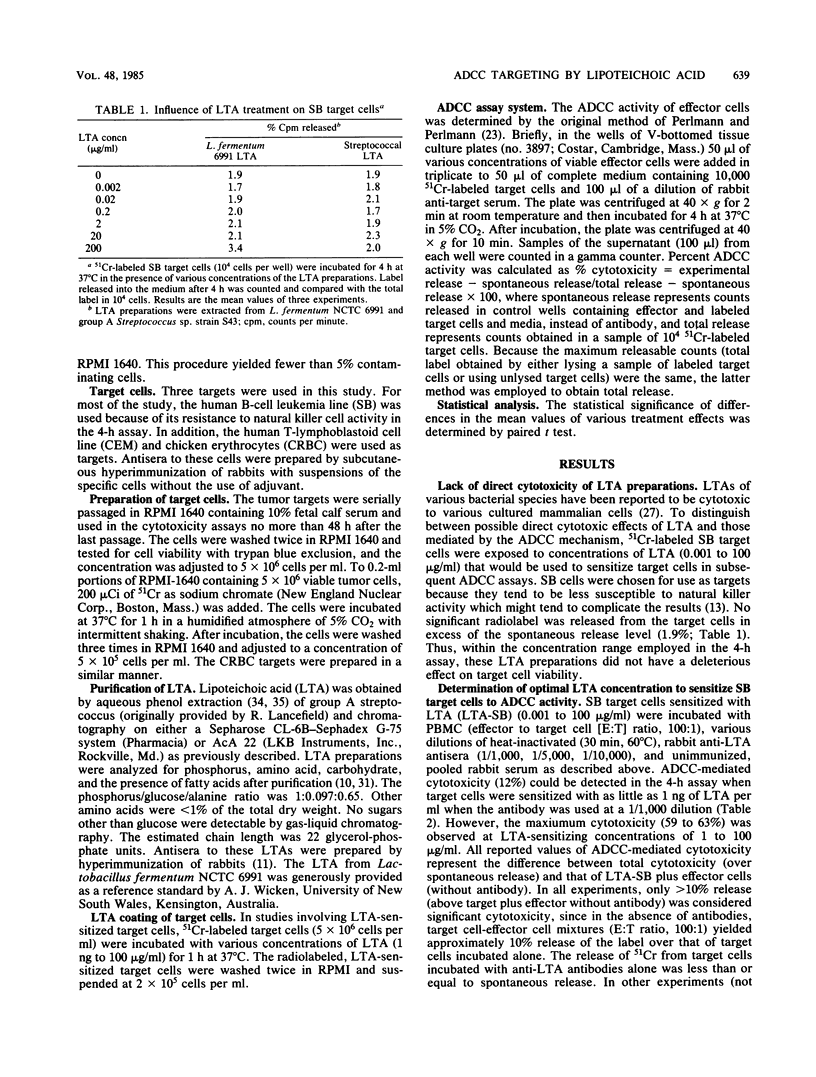
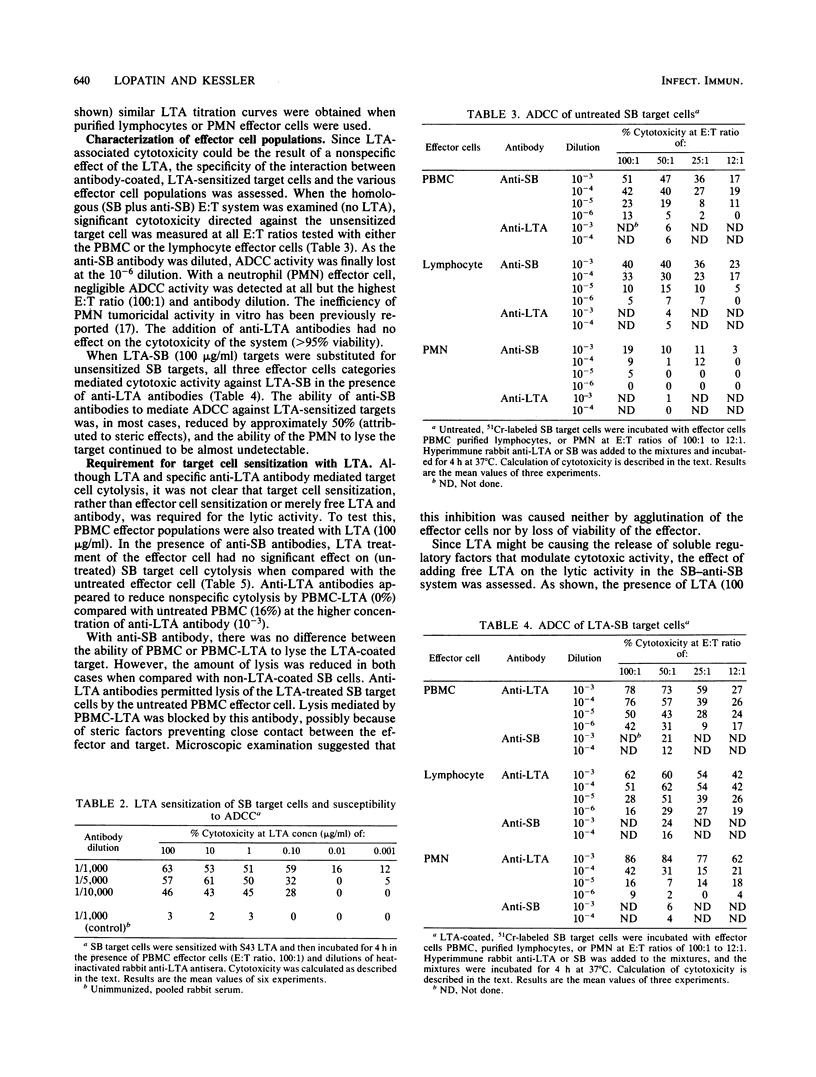
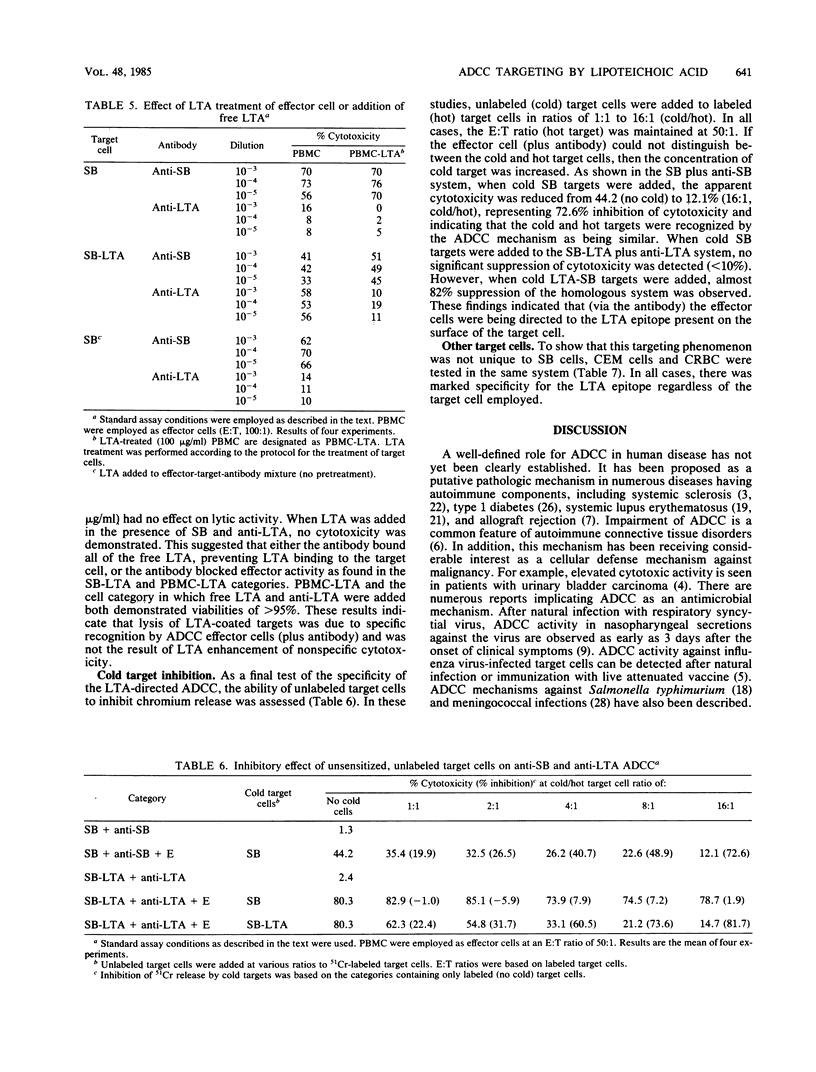
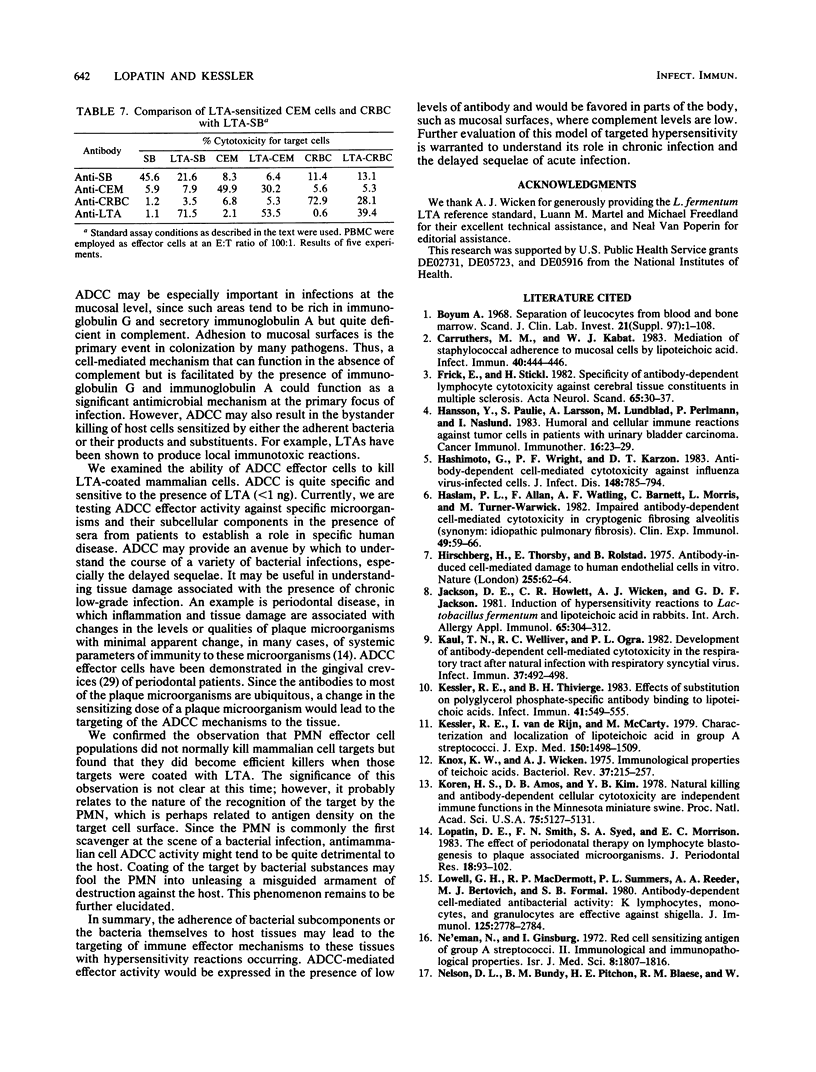
This study was performed to determine whether antibody-dependent cellular cytotoxicity (ADCC) could be directed against mammalian cells sensitized with spontaneously adhering bacterial substances. 51Cr-labeled SB leukemia cells were incubated with purified S43 group A streptococcal lipoteichoic acid (LTA; 0.001 to 100 micrograms/ml). Purified leukocyte ADCC effector cells were added to the LTA-coated target cells at various effector-to-target ratios (100:1 to 12:1), followed by the addition of rabbit anti-LTA. After incubation for 4 h, target cell lysis was calculated based on the release of label into the medium. As little as 1 ng of LTA per ml was sufficient to sensitize the target cells to ADCC lysis (12%); however, concentrations above 0.1 micrograms/ml generally resulted in 60 to 80% lysis. LTA alone was not cytotoxic to these target cells. Targeting did not occur if effector cells were sensitized or if free LTA was added to the medium. Specificity was demonstrated by cold-target inhibition, which showed that anti-LTA cytotoxicity could be inhibited only by unlabeled, LTA-treated target cells but not by cold SB cells alone. The findings indicate that certain soluble bacterial components, when bound to mammalian cells in the presence of specific antibody, can target ADCC effectors to these cells. This mechanism may be an important factor in the delayed sequelae of bacterial infections.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carruthers M. M., Kabat W. J. Mediation of staphylococcal adherence to mucosal cells by lipoteichoic acid. Infect Immun. 1983 Apr;40(1):444–446. doi: 10.1128/iai.40.1.444-446.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick E., Stickl H. Specificity of antibody-dependent lymphocyte cytotoxicity against cerebral tissue constituents in multiple sclerosis. Studies with basic protein of myelin, encephalitogenic peptide, cerebrosides and gangliosides. Acta Neurol Scand. 1982 Jan;65(1):30–37. doi: 10.1111/j.1600-0404.1982.tb03058.x. [DOI] [PubMed] [Google Scholar]
- Hansson Y., Paulie S., Larsson A., Lundblad M. L., Perlmann P., Näslund I. Humoral and cellular immune reactions against tumor cells in patients with urinary bladder carcinoma. Correlation between direct and antibody-dependent cell-mediated cytotoxicity. Cancer Immunol Immunother. 1983;16(1):23–29. doi: 10.1007/BF00199901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto G., Wright P. F., Karzon D. T. Antibody-dependent cell-mediated cytotoxicity against influenza virus-infected cells. J Infect Dis. 1983 Nov;148(5):785–794. doi: 10.1093/infdis/148.5.785. [DOI] [PubMed] [Google Scholar]
- Haslam P. L., Allan F., Watling A. F., Barrett C., Morris L., Turner-Warwick M. Impaired antibody-dependent cell-mediated cytotoxicity in cryptogenic fibrosing alveolitis (synonym: idiopathic pulmonary fibrosis). Clin Exp Immunol. 1982 Jul;49(1):59–66. [PMC free article] [PubMed] [Google Scholar]
- Hirschberg H., Thorsby E., Rolstad B. Antibody-induced cell-mediated damage to human endothelial cells in vitro. Nature. 1975 May 1;255(5503):62–64. doi: 10.1038/255062b0. [DOI] [PubMed] [Google Scholar]
- Jackson D. E., Howlett C. R., Wicken A. J., Jackson G. D. Induction of hypersensitivity reactions to Lactobacillus fermentum and lipoteichoic acid in rabbits. Part II. Int Arch Allergy Appl Immunol. 1981;65(3):304–312. doi: 10.1159/000232770. [DOI] [PubMed] [Google Scholar]
- Kaul T. N., Welliver R. C., Ogra P. L. Development of antibody-dependent cell-mediated cytotoxicity in the respiratory tract after natural infection with respiratory syncytial virus. Infect Immun. 1982 Aug;37(2):492–498. doi: 10.1128/iai.37.2.492-498.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., Thivierge B. H. Effects of substitution on polyglycerol phosphate-specific antibody binding to lipoteichoic acids. Infect Immun. 1983 Aug;41(2):549–555. doi: 10.1128/iai.41.2.549-555.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., van de Rijn I., McCarty M. Characterization and localization of the enzymatic deacylation of lipoteichoic acid in group A streptococci. J Exp Med. 1979 Dec 1;150(6):1498–1509. doi: 10.1084/jem.150.6.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Immunological properties of teichoic acids. Bacteriol Rev. 1973 Jun;37(2):215–257. doi: 10.1128/br.37.2.215-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren H. S., Amos D. B., Kim Y. B. Natural killing and antibody-dependent cellular cytotoxicity are independent immune functions in the Minnesota miniature swine. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5127–5131. doi: 10.1073/pnas.75.10.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatin D. E., Smith F. N., Syed S. A., Morrison E. C. The effect of periodontal therapy on lymphocyte blastogenesis to plaque associated microorganisms. J Periodontal Res. 1983 Jan;18(1):93–102. doi: 10.1111/j.1600-0765.1983.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Lowell G. H., MacDermott R. P., Summers P. L., Reeder A. A., Bertovich M. J., Formal S. B. Antibody-dependent cell-mediated antibacterial activity: K lymphocytes, monocytes, and granulocytes are effective against shigella. J Immunol. 1980 Dec;125(6):2778–2784. [PubMed] [Google Scholar]
- Ne'eman N., Ginsburg I. Red cell-sensitizing antigen of group A streptococci. II. Immunological and immunopathological properties. Isr J Med Sci. 1972 Nov;8(11):1807–1816. [PubMed] [Google Scholar]
- Nelson D. L., Bundy B. M., Pitchon H. E., Blaese R. M., Strober W. The effector cells in human peripheral blood mediating mitogen-induced cellular cytotoxicity and antibody-dependent cellular cytotoxicity. J Immunol. 1976 Nov;117(5 Pt 1):1472–1481. [PubMed] [Google Scholar]
- Nencioni L., Villa L., Boraschi D., Berti B., Tagliabue A. Natural and antibody-dependent cell-mediated activity against Salmonella typhimurium by peripheral and intestinal lymphoid cells in mice. J Immunol. 1983 Feb;130(2):903–907. [PubMed] [Google Scholar]
- Norris D. A., Ryan S. R., Fritz K. A., Kubo M., Tan E. M., Deng J. S., Weston W. L. The role of RNP, Sm, and SS-A/Ro-specific antisera from patients with lupus erythematosus in inducing antibody-dependent cellular cytotoxicity (ADCC) of targets coated with nonhistone nuclear antigens. Clin Immunol Immunopathol. 1984 Jun;31(3):311–320. doi: 10.1016/0090-1229(84)90084-9. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Jefferson W., Campbell G. L. Cell membrane-binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975 May 1;141(5):990–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning C. A., Hughes P., Rowell N. R. The production of antibody-dependent cellular cytotoxicity by immune complexes in systemic lupus erythematosus. J Clin Lab Immunol. 1984 Mar;13(3):123–127. [PubMed] [Google Scholar]
- Penning C. A., Wright J. K., Ashby J. C., Cunningham J., Rowell N. R., Hughes P. Serum-induced enhancement of peripheral blood mononuclear cell-mediated cytotoxicity towards human target cells in systemic sclerosis. J Clin Lab Immunol. 1983 Oct;12(2):77–81. [PubMed] [Google Scholar]
- Perlmann P., Perlmann H. Contactual lysis of antibody-coated chicken erythrocytes by purified lymphocytes. Cell Immunol. 1970 Sep;1(3):300–315. doi: 10.1016/0008-8749(70)90051-1. [DOI] [PubMed] [Google Scholar]
- Petz L. D., Sharp G. C., Cooper N. R., Irvin W. S. Serum and cerebral spinal fluid complement and serum autoantibodies in systemic lupus erythematosus. Medicine (Baltimore) 1971 Jul;50(4):259–275. doi: 10.1097/00005792-197107000-00002. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Thompson R. E. Pulmonary host defenses. I. Analysis of protein and lipids in bronchial secretions and antibody responses after vaccination with pseudomonas aeruginosa. J Immunol. 1973 Aug;111(2):358–368. [PubMed] [Google Scholar]
- Sensi M., Pozzilli P., Cudworth A. G. Antibody-dependent and natural killer cytotoxicity in type 1 diabetes. Acta Diabetol Lat. 1981 Oct-Dec;18(4):339–344. doi: 10.1007/BF02042818. [DOI] [PubMed] [Google Scholar]
- Simpson W. A., Dale J. B., Beachey E. H. Cytotoxicity of the glycolipid region of streptococcal lipoteichoic acid for cultures of human heart cells. J Lab Clin Med. 1982 Jan;99(1):118–126. [PubMed] [Google Scholar]
- Smith L. F., Lowell G. H. Antibody-dependent cell-mediated antibacterial activity of human mononuclear cells. II. Immune specificity of antimeningococcal activity. J Infect Dis. 1980 Jun;141(6):748–751. doi: 10.1093/infdis/141.6.748. [DOI] [PubMed] [Google Scholar]
- Sonis S. T., Mirando D., Stelos P., Lamster I. B. Capacity of human oral leucocytes to mediate antibody-dependent cell-mediated cytotoxicity. Arch Oral Biol. 1979;24(3):235–237. doi: 10.1016/0003-9969(79)90147-x. [DOI] [PubMed] [Google Scholar]
- Tagliabue A., Nencioni L., Villa L., Keren D. F., Lowell G. H., Boraschi D. Antibody-dependent cell-mediated antibacterial activity of intestinal lymphocytes with secretory IgA. Nature. 1983 Nov 10;306(5939):184–186. doi: 10.1038/306184a0. [DOI] [PubMed] [Google Scholar]
- Vercellotti G. M., Lussenhop D., Peterson P. K., Furcht L. T., McCarthy J. B., Jacob H. S., Moldow C. F. Bacterial adherence to fibronectin and endothelial cells: a possible mechanism for bacterial tissue tropism. J Lab Clin Med. 1984 Jan;103(1):34–43. [PubMed] [Google Scholar]
- Waltersdorff R. L., Fiedel B. A., Jackson R. W. Induction of nephrocalcinosis in rabbit kidneys after long-term exposure to a streptococcal teichoic acid. Infect Immun. 1977 Sep;17(3):665–667. doi: 10.1128/iai.17.3.665-667.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Gibbens J. W., Knox K. W. Comparative studies on the isolation of membrane lipoteichoic acid from Lactobacillus fermenti. J Bacteriol. 1973 Jan;113(1):365–372. doi: 10.1128/jb.113.1.365-372.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975 Mar 28;187(4182):1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr, Van de Rijn I., Reid H., Poon-King T., Zabriskie J. B. Lymphocyte cell subpopulations during acute post-streptococcal glomerulonephritis: cell surface antigens and binding of streptococcal membrane antigens and C-reactive protein. Clin Exp Immunol. 1981 Nov;46(2):397–405. [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., Kessler R. E. Chemical analysis of changes in membrane composition during growth of Streptococcus pyogenes. Infect Immun. 1979 Dec;26(3):883–891. doi: 10.1128/iai.26.3.883-891.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


