Abstract
Respiratory tract infection, most often involving opportunistic bacterial species with broad-spectrum antibiotic resistance, is the primary cause of death in persons with cystic fibrosis (CF). Species within the Burkholderia cepacia complex are especially problematic in this patient population. We investigated a novel surfactant-stabilized oil-in-water nanoemulsion (NB-401) for activity against 150 bacterial isolates recovered primarily from CF respiratory tract specimens. These specimens included 75 Burkholderia isolates and 75 isolates belonging to other CF-relevant species including Pseudomonas, Achromobacter, Pandoraea, Ralstonia, Stenotrophomonas, and Acinetobacter. Nearly one-third of the isolates were multidrug resistant, and 20 (13%) were panresistant based on standard antibiotic testing. All isolates belonging to the same species were genotyped to ensure that each isolate was a distinct strain. The MIC90 of NB-401 was 125 μg/ml. We found no decrease in activity against multidrug-resistant or panresistant strains. MBC testing showed no evidence of tolerance to NB-401. We investigated the activity of NB-401 against a subset of strains grown as a biofilm and against planktonic strains in the presence of CF sputum. Although the activity of NB-401 was decreased under both conditions, the nanoemulsion remained bactericidal for all strains tested. These results support NB-401's potential role as a novel antimicrobial agent for the treatment of infection due to CF-related opportunistic pathogens.
For reasons that are not well understood, persons with cystic fibrosis (CF) are prone to chronic infection of the respiratory tract, which ultimately leads to pulmonary failure, the primary cause of death in this patient population. Pathogenic bacterial species such as Staphylococcus aureus and Haemophilus influenzae are commonly involved in infection in young CF patients, while a growing number of opportunistic species causes infection in older individuals. Chief among these species is Pseudomonas aeruginosa, which infects the majority of adults with CF. Several other species, although less frequently recovered in specimens from CF patients, are particularly problematic due to their multidrug-resistant phenotypes. These species include Achromobacter xylosoxidans and Stenotrophomonas maltophilia as well as several Ralstonia and Pandoraea species. The frequency of respiratory tract infection by these species increases with patient age, posing a significant health risk to the growing number of CF patients surviving to adulthood.
Species of the Burkholderia cepacia complex are especially troublesome in CF, where they are associated with increased rates of morbidity and mortality (35). Burkholderia species are also among the most antimicrobial-resistant bacterial species encountered in human infection. Numerous reports document the survival of Burkholderia in antiseptic solutions including alcohol, chlorhexidine, benzalkonium chloride, and povidone-iodine and in disinfectants such as quaternary ammonium compounds (61). Antimicrobial therapy of human infection due to Burkholderia is severely limited by constitutive and inducible broad-spectrum resistance that is exhibited by most strains (33, 38, 47). Most Burkholderia species also appear to be capable of biofilm formation, which likely also impedes antimicrobial therapy in infected patients (9, 54). Finally, the treatment of respiratory tract infection in CF is complicated by the presence of infecting organisms in highly viscous sputum, which presents a barrier to antibiotic delivery. Clearly, there is a need for new antimicrobial agents that may be effective in treating infection due to Burkholderia and other multiresistant bacterial species in persons with CF.
Nanoemulsions are water-in-vegetable-oil formulations produced by mixing the water-immiscible liquid phase into an aqueous phase by high-stress mechanical extrusion (2). A novel nonionic surfactant nanoemulsion comprised of tributyl phosphate, soybean oil, and Triton X-100 emulsified in water was previously shown to have broad-range antimicrobial activity (25). This included bactericidal activity against H. influenzae, Bacillus cereus, Neisseria gonorrhoeae, Streptococcus pneumoniae, and Vibrio cholerae as well as viricidal activity against herpes simplex virus, influenza A virus, and vaccinia virus. This compound also demonstrated sporicidal activity against Bacillus species and remarkably low toxicity in experimental animals (24, 25). Other nanoemulsions showed inhibitory activity against Candida parapsilosis and clinically important filamentous fungi including Microsporum spp., Trichophyton mentagrophytes, Trichophyton rubrum, Aspergillus fumigatus, Fusarium oxysporum, and Epidermophyton floccosum (43).
In the present study, we examined the in vitro activities of a novel nanoemulsion compound against a panel of multidrug-resistant bacterial isolates recovered from patients with CF. In addition to microtiter broth dilution MIC testing, we assessed nanoemulsion activity against a representative subset of strains grown as a biofilm and against planktonic strains in the presence of CF sputum.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
One hundred fifty isolates were studied. These included 75 Burkholderia isolates and 75 isolates belonging to other CF-relevant species including P. aeruginosa, A. xylosoxidans, S. maltophilia, Acinetobacter species, Pandoraea species (P. apista, P. pnomenusa, P. pulmonicola, P. norimburgensis, and P. sputorum), and Ralstonia species (R. mannitolilytica and R. pickettii). One hundred forty-five clinical isolates were obtained from the Burkholderia cepacia Research Laboratory and Repository (BcRLR) (University of Michigan, Ann Arbor, MI). These isolates were recovered from 142 individuals between September 1997 and October 2007 and were referred to the BcRLR for analysis from 62 CF treatment centers in the United States. The remaining five strains included the environmental isolates Burkholderia multivorans ATCC 17616 (American Type Culture Collection, Manassas, VA), P. norimburgensis LMG 18379T (BCCM/LMG Bacteria Collection, Laboratorium voor Micrbiologie Gent, Universiteit Gent, Ghent, Belgium), and Burkholderia pyrrocinia HI3642 (BcRLR collection) and the clinical type strains B. cenocepacia LMG 16656T (also referred to as J2315) (60) and P. pulmonicola LMG 18106T. Of the 147 clinical isolates, 134 (91%) were recovered from persons with CF: 114 (78%) were from sputum culture, with the remainder from throat swab (n = 17), endotracheal suction/tracheal aspiration (n = 5), blood (n = 5), bronchial lavage (n = 3), and maxillary sinus, peritoneal cavity, and epiglottis (one each) samples. Forty-nine (33%) of the 150 isolates were defined as being multidrug resistant (resistant to all drugs tested in two of three antibiotic classes, lactams including carbapenems, aminoglycosides, and quinolones) (55) based on susceptibility testing performed at the referring microbiology laboratory; 20 (41%) of these were panresistant. Seventy-two (48%) of the remaining isolates were susceptible to at least one antibiotic, and susceptibility testing results were unavailable for 29 (19%) isolates. All isolates were identified to the species level at the BcRLR by polyphasic analyses using phenotypic and genotypic assays as described previously (48). The exception was Acinetobacter, which was identified only to the genus level due to the lack of definitive species-specific assays. All isolates were also subjected to repetitive extragenic element-PCR typing using primer BOX A1R as previously described (15) to ensure that all 150 isolates were genotypically distinct. Bacteria were stored at −80°C in skim milk or Luria-Bertani broth with 15% glycerol and recovered from frozen stock overnight at 37°C on Mueller-Hinton (MH) agar.
Nanoemulsion.
Nanoemulsion NB-401 was supplied by NanoBio Corp. (Ann Arbor, MI) and was manufactured by emulsification of cetylpyridinium chloride (CPC), poloxamer 407, and ethanol in water with superrefined soybean oil using a high-speed emulsifier. The resultant droplets had a mean particle diameter of 400 nm. The surfactants and food substances used were “generally recognized as safe” by the FDA and manufactured in accordance with good manufacturing practices. The concentration of CPC was used as a surrogate for the amount of NB-401 used experimentally. NB-401 was stable for no less than 12 months at temperatures up to 40°C.
NB-401 activity against planktonic bacteria.
The MICs of NB-401 were determined by using a modification of the Clinical and Laboratory Standards Institute (CLSI)-approved microtiter serial dilution method (14). NB-401 was diluted to a concentration of 2 mg/ml (of CPC) in MH broth supplemented with 7% NaCl and 20 mM EDTA. Serial twofold dilutions of this preparation were made in unsupplemented MH broth and aliquoted into 96-well flat-bottom microtiter plates (100 μl/well). Bacteria from overnight growth on MH agar were suspended in MH broth to a 0.5 McFarland turbidity standard (absorbance of 0.08 to 0.13 at 625 nm), further diluted 1:100 in MH broth, and added (5 μl/well) to the NB-401 serial dilution wells. Appropriate controls, including wells with bacteria but no NB-401 and wells with NB-401 dilutions but no bacteria, were included on each plate. Microtiter plates were shaken briefly, and 1 μl was removed from wells containing bacteria but no NB-401, diluted in 1 ml of MH broth, plated onto MH agar (100 μl), and incubated for 24 to 48 h at 37°C to confirm that initial inoculums were ≥105 CFU. Microtiter plates were then incubated at 37°C without shaking. To determine minimal bactericidal concentrations (MBCs), 10 μl was removed from each well after overnight growth, spread onto MH agar, and incubated at 37°C. Colonies were enumerated 24 h later, and MBCs were recorded as the NB-401 concentrations with a 3-log decrease in CFU/ml compared to the initial inoculums. Because NB-401 is opaque, 10 μl of resazurin (R&D Systems, Minneapolis, MN) was added to each well, and microtiter plates were shaken briefly, covered with foil, and incubated at 37°C without shaking. Resazurin, a nonfluorescing blue dye, is reduced to resorufin, a fluorescing pink dye, in the presence of actively metabolizing cells. Therefore, MICs were recorded the next day as the lowest concentrations of NB-401 in which the wells remained blue. MIC results were further quantified by measuring the fluorescence generated by the reduction product resorufin on a spectrofluorometer at 560 nm excitation/590 nm emission. The change in metabolic activity for treated bacteria was calculated as follows: (fluorescence of the visual MIC well − fluorescence of the well with the equivalent concentration of NB-401 without bacteria)/(fluorescence of the well containing bacteria but no NB-401 − fluorescence of the well containing medium only) × 100 (56).
NB-401 activity against bacteria grown in biofilm.
To identify biofilm-forming isolates, bacteria were grown overnight in tryptic soy broth, adjusted to a 0.5 McFarland turbidity standard, and further diluted 1:10 in MH broth, and 100 μl was seeded in triplicate in internal wells of 96-well flat-bottom microtiter plates. Negative control wells without bacteria were included. To minimize evaporation, the remaining wells were filled with MH broth, and the plate was covered with plastic wrap before incubation for 48 h at 37°C without shaking. Wells were gently washed twice with phosphate-buffered saline (PBS) (pH 7.4), dried for 2 h at 37°C, and then stained with 1% crystal violet in water for 15 min. Stained wells were washed three times with PBS, and crystal violet was eluted in absolute methanol. After incubation for 5 min at room temperature, the solubilized crystal violet was transferred onto a new microtiter plate and scanned at 590 nm in a spectrophotometer. A biofilm-forming isolate was defined as one in which the average absorbance of the three wells was greater than the average absorbance of the negative control wells plus 3 standard deviations (53).
To test nanoemulsion activity against biofilm cultures, isolates shown to produce biofilm by crystal violet staining were grown in triplicate for 48 h as described above. Wells were gently washed twice with PBS before the addition of NB-401, serially diluted as described above for planktonic MIC testing. After overnight incubation at 37°C, wells were again washed twice with PBS, and 100 μl of 10% resazurin in MH broth was added to wells. Plates were covered in plastic wrap, covered with foil, and again incubated overnight at 37°C without shaking. Plates were visually inspected the next day, and minimum biofilm inhibitory concentrations (MBICs) were recorded as the lowest concentrations of NB-401 in which the wells remained blue. To calculate minimum biofilm eradication concentrations (MBECs), the biofilm was resuspended in the same wells used for MBIC testing by shaking the microtiter plate and then scraping the sides of the wells with a pipette tip. Ten microliters was removed from each well, spotted onto MH agar plates, and incubated at 37°C. After overnight growth, MBECs were recorded as the lowest NB-401 concentrations without colony growth (11). To confirm initial inoculum concentrations and to quantify viable bacteria in biofilm-grown cultures, colonies were enumerated from 10-fold serial dilutions of the inoculating culture at a 0.5 McFarland standard and from the triplicate untreated positive control wells in which biofilm was resuspended by scraping for MBEC calculations.
NB-401 activity against bacteria in CF sputum.
Expectorated sputum, collected from CF patients during the course of routine care, was obtained from the University of Michigan Health System clinical microbiology laboratory and stored at −80°C. Equal volumes of sputum from 15 individuals were pooled, mechanically sheared using a Tissue Miser homogenizer (Fisher Scientific, Pittsburg, PA) at room temperature for 5 min at maximum speed, and then incubated in an 80°C water bath for 20 min. Processed sputum was divided into 10-ml aliquots, and 100 μl from each aliquot was plated onto MH agar and incubated for 48 h at 37°C to confirm sterility. Aliquots were stored at −80°C. To calculate MBCs in the presence of CF sputum, MBCs of NB-401 were determined as described above except that MH broth was replaced with the processed CF sputum preparation. The final concentration of CF sputum was 43%.
Time-kill analysis.
Four replicate microtiter plates, each containing twofold serial dilutions of NB-401, were prepared and inoculated with B. multivorans ATCC 17616 as described above for planktonic testing. Negative control wells without bacteria were included on each plate. Plates were incubated at 37°C, and 10-fold serial dilutions were plated in triplicate onto MH agar after 30 min, 60 min, 90 min, and 24 h. After a further 24 h of incubation at 37°C, colonies were enumerated and recorded as the average CFU/ml.
RESULTS
NB-401 activity against planktonic bacteria.
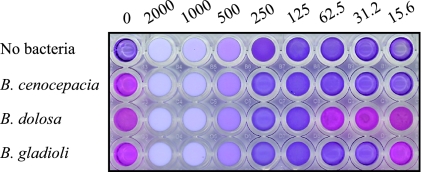
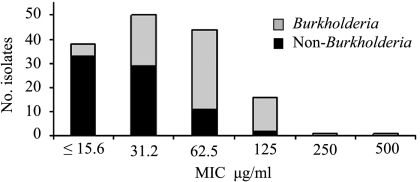
Due to the opaque white color of NB-401, the standard CLSI-approved microtiter serial dilution method was modified to include the addition of resazurin as an indicator of bacterial viability. NB-401 was tested in a concentration range of 15.6 to 2,000 μg/ml. MICs were defined as the lowest concentrations of NB-401 that did not produce color changes from blue to pink (Fig. 1). Comparison of these visual inflection points with fluorometric analyses showed that based on growth in untreated positive control wells, 63% of MIC wells had ≤1% metabolic activity, 91% had ≤5% metabolic activity, and 96% had ≤10% metabolic activity. The MIC results are shown in Table 1. All strains were inhibited by the concentrations of NB-401 tested. The MIC50 for the entire panel of 150 strains was 31.2 μg/ml; the MIC90 was 125 μg/ml. Thirty-eight strains (25%) were inhibited by the lowest concentration of NB-401 tested (15.6 μg/ml), and only a single strain each required a concentration of 250 μg/ml and 500 μg/ml for inhibition. NB-401 was slightly more active against non-Burkholderia strains (MIC50 of 31.2 μg/ml; MIC90 of 62.5 μg/ml) than Burkholderia strains (MIC50 of 62.5 μg/ml; MIC90 of 125 μg/ml) (Fig. 2). Activities were comparable across the 10 Burkholderia species tested. No difference was found in the activities of NB-401 against multidrug-resistant strains, strains susceptible to one or more antibiotics, or the subset of 29 strains without antibiotic susceptibility data (data not shown).
FIG. 1.
Representative microtiter serial dilution MIC assay. Numbers above the plate indicate the concentration (μg/ml CPC) of NB-401 added to each column of wells. The bacterial species added to wells in each row are indicated on the left. The MICs for these strains (top to bottom) are ≤15.6 μg/ml, 125 μg/ml, and 31.2 μg/ml.
TABLE 1.
In vitro activities of NB-401
| Species (no. of isolates tested) | MIC (μg/ml CPC)
|
||
|---|---|---|---|
| 50% | 90% | Range | |
| Burkholderia | |||
| B. cepacia (5) | 31.2-125 | ||
| B. multivorans (10) | 62.5 | 125 | 31.2-125 |
| B. cenocepacia (20) | 62.5 | 125 | ≤15.6-500 |
| B. stabilis (5) | ≤15.6-125 | ||
| B. vietnamiensis (5) | ≤15.6-62.5 | ||
| B. dolosa (5) | 62.5-125 | ||
| B. ambifaria (5) | 31.2-62.5 | ||
| B. anthina (5) | 31.2-62.5 | ||
| B. pyrrocinia (5) | 31.2-125 | ||
| B. gladioli (10) | 31.2 | 125 | ≤15.6-125 |
| P. aeruginosa (20) | 31.2 | 62.5 | ≤15.6-62.5 |
| A. xylosoxidans (10) | 31.2 | 62.5 | 31.2-62.5 |
| S. maltophilia (15) | ≤15.6 | 31.2 | ≤15.6-62.5 |
| Acinetobacter (10) | ≤15.6 | 125 | ≤15.6-125 |
| Pandoraea | |||
| P. apista (2) | 31.2 | ||
| P. norimbergensis (2) | 31.2 | ||
| P. pnomenusa (2) | 31.2 | ||
| P. pulmonicola (2) | 31.2-62.5 | ||
| P. sputorum (2) | 31.2-62.5 | ||
| Ralstonia | |||
| R. mannitolilytica (5) | ≤15.6-31.2 | ||
| R. pickettii (5) | ≤15.6 | ||
| Total (150) | 31.2 | 125 | ≤15.6-500 |
FIG. 2.
Distribution of NB-401 MICs for 150 Burkholderia and non-Burkholderia strains.
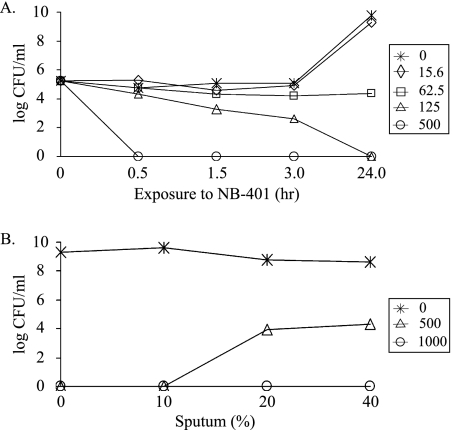
To evaluate the bactericidal activity of NB-401 against planktonic bacteria, MBCs were determined for a subset of 34 strains including 22 Burkholderia and 12 non-Burkholderia strains. All MBCs were within 1 dilution of the respective MICs for this subset of strains except for a single B. cenocepacia strain that had an MIC of 250 μg/ml and an MBC of 2,000 μg/ml (data not shown). A time-kill study of B. multivorans ATCC 17616 showed time- and concentration-dependent killing, with a 99% decrease in bacterial viability within 90 min at an NB-401 concentration two times greater than the MIC and complete killing within 30 min at concentrations eight times greater than the MIC (Fig. 3A).
FIG. 3.
Time-kill study of NB-401 activity and concentration-dependent inhibition of NB-401 activity by CF sputum. (A) B. multivorans ATCC 17616 was exposed to various concentrations of NB-401 around the MIC (62.5 μg/ml), and viable bacterial counts were determined at the times indicated. (B) B. multivorans ATCC 17616 was exposed to various concentrations of NB-401 in the presence of three concentrations of CF sputum for 24 h before viable bacterial counts were determined. Numbers and symbols in inset boxes indicate concentrations of NB-401 (μg/ml CPC) used.
NB-401 activity against bacteria grown in a biofilm.
To further assess the activity of NB-401, bacteria were grown as biofilms. Twelve biofilm-forming strains were identified from crystal violet staining of 25 strains from the test panel. The median increase in MBICs compared to the respective MICs of NB-401 for these strains was eightfold. Nine (75%) of the 12 strains showed decreased susceptibility, defined as at least a fourfold increase in the MBIC compared to the MIC (Table 2). Similar to planktonic bacteria, biofilm bacteria did not exhibit evidence of tolerance to NB-401; MBECs were identical to their respective MBICs for 10 of the 12 strains.
TABLE 2.
In vitro activities of NB-401 against biofilm bacteria and in the presence of CF sputuma
| Strain | Species | MIC (μg/ml CPC) | MBC (μg/ml CPC) | MBIC (μg/ml CPC) | MBEC (μg/ml CPC) | SMBC (μg/ml CPC) |
|---|---|---|---|---|---|---|
| AU8042 | B. multivorans | 125 | 125 | 1,000 | 1,000 | 1,000 |
| AU10398b | B. multivorans | 62.5 | 62.5 | 500 | 500 | 250 |
| ATCC 17616 | B. multivorans | 62.5 | 62.5 | 1,000 | 1,000 | 1,000 |
| AU10321 | B. cenocepacia | 31.2 | 31.2 | 1,000 | 1,000 | 1,000 |
| LMG16656T | B. cenocepacia | 62.5 | 125 | 500 | 1,000 | 1,000 |
| AU4757 | B. stabilis | 125 | 125 | 500 | 500 | 500 |
| AU10529 | B. gladioli | ≤15.6 | ≤15.6 | 62.5 | 62.5 | 31.2 |
| AU13206 | A. xylosoxidans | 62.5 | 125 | 500 | 2,000 | 1,000 |
| AU12828 | P. aeruginosa | 31.2 | 31.2 | 1,000 | 1,000 | 500 |
| AU8215a | P. aeruginosa | 31.2 | 31.2 | 31.2 | 31.2 | 62.5 |
| AU12914 | R. pickettii | ≤15.6 | ≤15.6 | ≤15.6 | ≤15.6 | 31.2 |
| AU4194 | S. maltophilia | ≤15.6 | ≤15.6 | 31.2 | 31.2 | 31.2 |
Values represent μg/ml CPC. SMBC is MBC in the presence of 43% CF sputum.
NB-401 activity against bacteria in CF sputum.
To model the CF pulmonary microenvironment more closely, the activity of NB-401 was tested against the 12 biofilm-forming strains in the presence of CF sputum. Under planktonic conditions, the MBCs of NB-401 for all 12 strains increased in the presence of 43% sputum (the highest sputum concentration achievable in the microtiter assay) compared to the respective MBCs (Table 2). Nevertheless, NB-401 showed antibacterial activity against all strains in the presence of sputum. MICs in the presence of sputum were not determined since sputum was inherently fluorescent and masked the fluorescence emitted by the reduction of resazurin. CF sputum inhibited the activity of NB-401 against planktonic bacteria in a concentration-dependent manner (Fig. 3B).
DISCUSSION
Although the bacterial species that we included in this study are generally not virulent in healthy persons, these opportunists can cause severe and chronic respiratory tract infections in individuals with CF. Unremitting infection and the associated inflammation result in progressive lung disease that culminates in pulmonary failure, the leading cause of death for CF patients. Effective therapy of CF pulmonary infection is severely limited by the broad-spectrum antimicrobial resistance exhibited by these species, which are among the most drug-resistant bacteria encountered in human infection. The site of infection in CF presents another important obstacle to effective therapy. Infecting bacteria reside primarily within the airway lumen in sputum, the airway epithelial surface fluid, and the bronchial mucosa (3, 37, 49, 58). The penetration of systemically delivered antimicrobials to this infected site is generally poor. Treatment is further hampered by bacterial biofilm formation, which is believed to occur in the airways of infected patients (51), and by the exceptionally viscous secretions that characterize the CF respiratory tract (40, 52). These challenges have driven an increase in recent efforts to develop antimicrobials for topical (i.e., inhalational) use in CF (8, 21, 27).
The surfactant-stabilized oil-in-water nanoemulsion that we investigated in this study is similar to others that were previously found to be bactericidal against gram-positive bacteria and spores as well as some gram-negative bacilli and enveloped viruses (13, 24, 25, 42). The mechanism of bacterial and viral killing is believed to involve the fusion of the emulsion with microorganism lipid membranes, leading to rapid osmotic disruption and cell lysis (23). The electrostatic attraction provided by the cationic surface charge of CPC-based nanoemulsions appears to overcome the lipopolysaccharide-mediated resistance of gram-negative bacteria to neutral and anionic detergents (23). The nanoemulsion used in this study was not selected for specific activity against the bacterial species included in our test panel. However, in preliminary studies, we found that the bactericidal activity of NB-401 against gram-negative bacteria was enhanced by the addition of EDTA, which likely chelates divalent cations that stabilize outer membrane lipopolysaccharide, thereby facilitating interactions with the cationic emulsion. This interaction most likely leads to membrane permeabilization and lysis as well as the augmentation of the transmembrane diffusion of macromolecules (1, 26, 32, 59).
Inhaled nebulized hypertonic saline was recently shown to have clinical benefit in the management of CF (18), possibly resulting from the osmotic restoration of airway surface fluid and improvement of airway mucus rheological properties (19). The addition of 7% saline did not decrease the bactericidal activity of NB-401. In preliminary studies, we found NB-401 to be stable after nebulization (using a PARI LC Plus nebulizer) in 7% saline and well tolerated by mice as a single inhaled dose (data not shown).
We focused our investigation of NB-401 on species within the B. cepacia complex, as infection with these species is particularly refractory to antimicrobial therapy and is associated with increased rates of morbidity and mortality in CF (7). Importantly, infection with members of the B. cepacia complex is also regarded by many CF care centers as an absolute contraindication to lung transplantation (34). Because B. multivorans and B. cenocepacia account for the majority of B. cepacia complex infections in CF (48), we included relatively more strains from these two species. We also included Burkholderia gladioli, which, although not a member of the B. cepacia complex, is being recovered from CF patients with increasing frequency (4). The numbers of strains included in our test panel from the remaining species were also chosen to reflect the relative proportion of these species recovered from CF patients. The exception is Acinetobacter, which, although currently infrequently recovered in CF, appears to be an emerging pathogen in this patient population. The majority (94%) of isolates in the test panel were recovered from cultures of respiratory specimens from persons with CF. The panel also included one representative isolate from each of five previously described so-called epidemic lineages, each of which has been identified as infecting multiple CF patients. These included the B. cenocepacia ET12 (29), PHDC (12), and Midwest (16) lineages as well as the B. multivorans OHBM lineage (5) and the B. dolosa SLC6 lineage (5). Finally, to ensure a stringent test of the activity of NB-401, one-third of the isolates in the test panel were multidrug resistant; 20 of these were panresistant. To avoid duplicate testing of the same strain, all isolates were confirmed as being distinct strains by genotyping analyses.
NB-401 showed very good activity in vitro against the strains studied. With the exception of two B. cenocepacia strains, all strains in the test panel were inhibited by an NB-401 concentration of ≤125 μg/ml, or a 1:16 dilution of the initial formulation; 59% of strains were inhibited by a concentration of ≤31.2 μg/ml, a 1:64 dilution of NB-401. NB-401 did not show decreased activity against multidrug-resistant (MIC90 of 125 μg/ml) or panresistant (MIC90 of 62.5 μg/ml) strains. In general, NB-401 showed slightly less activity against Burkholderia species than the other species examined, with 16 of the 18 highest MICs found among Burkholderia strains. Conversely, 33 of the 38 lowest MICs (15.6 μg/ml) were found among non-Burkholderia species. We observed no striking differences in NB-401 activity among the 10 Burkholderia species examined, although the two strains requiring the highest MICs (250 μg/ml and 500 μg/ml) were both B. cenocepacia. Among the non-Burkholderia species, NB-401 was most active against Ralstonia strains (MIC90 of ≤15.6 μg/ml) and relatively less active against Acinetobacter strains (MIC90 of 125 μg/ml). We found no evidence of tolerance to NB-401 among a subset of 34 strains for which both MICs and MBCs were determined. NB-401 killing of planktonically grown bacteria was time and concentration dependent; at a concentration eight times greater than the MIC, complete killing was achieved within 30 min. This suggests that a relatively brief exposure of bacteria to NB-401 could result in a significant decrease in levels of viable bacteria during therapy.
While we found NB-401 to be highly active against a rigorously assembled panel of CF-associated bacteria, the relevance of standard susceptibility testing of planktonically grown bacteria to the treatment of infections involving bacteria within biofilms has been questioned recently. Several species involved in CF infection, including P. aeruginosa and Burkholderia species, have the capacity to produce biofilms in vitro, and emerging evidence strongly suggests that biofilm formation within the airways of infected patients contributes to disease progression and persistence of infection (20, 39, 50, 51). Furthermore, sessile bacteria within biofilms demonstrate increased antimicrobial resistance relative to their planktonic counterparts (9, 17, 41, 54). We therefore sought to assess the activity of NB-401 against bacteria grown in vitro as biofilms. Using a relatively strict definition of in vitro biofilm formation to ensure a stringent test of NB-401 activity, 12 biofilm-forming strains representing several species and a range of planktonic susceptibilities to NB-401 were identified. As there is currently no standardized protocol for susceptibility testing of bacteria in biofilm, we considered the merits of various previously reported methods (9-11, 17, 41, 44-46, 53, 57) and tested a number of variables to develop a combination of steps that we believed were best suited for our study, particularly considering the opacity of the nanoemulsion. These steps included comparing inoculum volumes and concentrations, peg versus well surfaces for biofilm growth, durations of biofilm growth, spectrophotometric versus colorimetric measurements, and various methods to suspend biofilm bacteria after treatment including sonication, centrifugation, and scraping of surfaces to release cells (data not shown). Although the MBIC and MBEC of NB-401 were increased compared to the respective MIC and MBC for each strain tested (median eightfold increase in MBIC compared to MIC), all 12 strains were inhibited or killed by NB-401 when grown as biofilms. Only a single strain required undiluted NB-401 (2,000 μg/ml) for the eradication of viable biofilm bacteria. We assessed relative biomasses among the biofilm-forming strains spectrophotometrically with crystal violet staining and observed no correlation between biomass and MBIC/MBEC results (data not shown). In fact, although B. gladioli strain AU10529 produced the greatest biomass among the 12 strains tested, the MBIC and MBEC of NB-401 for this strain were relatively low (both 62.5 μg/ml). Conversely, B. cenocepacia strain J2315 produced relatively little biomass yet required an NB-401 MBEC of 1,000 μg/ml. Similarly, we observed no correlation between colony counts from untreated biofilm wells and MBIC/MBEC results (data not shown). Intra- and interexperimental reproducibilities of colony counts from the untreated biofilm controls validated the uniformity of the scraping method and the overall biofilm assay.
CF sputum, like biofilm, is reported to antagonize the activity of antibacterial drugs (28). Glycoproteins and high-molecular-weight DNA are present at elevated levels, resulting in exceptionally viscous sputum that provides a physical barrier protecting bacteria. In addition, these macromolecules bind and sequester antibiotics while small cationic molecules, and the decreased pH of CF sputum blocks drug penetration into bacteria and reduces drug bioactivity. The strategy of increasing drug dosing to overcome these obstacles is limited by drug toxicity. To assess the impact of sputum on the antibacterial activity of NB-401, we repeated the standard planktonic testing for the 12 biofilm-forming strains in the presence of CF sputum. We used a mixture of sputum from 15 CF patients to avoid interpatient variation in macromolecule and high-molecular-weight DNA compositions and in ionic conditions and applied mechanical shearing only to minimize changes to the native microenvironment (22). The activity of NB-401 against bacteria suspended in medium containing 43% sputum (the maximum sputum concentration achieved in our test system) was decreased with bactericidal concentrations 2- to 32-fold greater than the respective planktonic MBCs without sputum. The sputum MBCs were identical to or within 1 dilution of the MBECs obtained with biofilm-grown bacteria. Although the activity of NB-401 was similarly antagonized by both CF sputum and biofilm growth, it remained bactericidal for all the strains tested under both test conditions.
In summary, the results of this study support a potential role for NB-401 as an antimicrobial treatment for infection due to CF-related opportunistic pathogens. Most strains tested, including many multi- and panresistant strains, were inhibited in vitro by ≤125 μg/ml of NB-401 (a 16-fold dilution of the nanoemulsion). This emulsion was rapidly bactericidal and was active against bacteria whether grown planktonically, as a biofilm, or in the presence of CF sputum. In ongoing work, we have found nanoemulsions to be exceptionally stable, unchanged after nebulization, and broadly microbicidal. Importantly, we have not observed the development of resistance to NB-401 by any bacterial species examined to date. Comprehensive phase II clinical safety, tolerability, and pharmacokinetic studies of similar nanoemulsion formulations for use as topical therapeutics for herpes labilis and onychomycosis have shown these formulations (NB-001 and NB-002, respectively) to be safe and well tolerated, as described previously (30, 31). Nasal application of similar nanoemulsions as vaccine delivery agents was well tolerated and did not induce inflammation in mice (6, 42), and a recent preclinical safety evaluation of a nanoemulsion vaccine adjuvant similarly showed no inflammation, cytotoxicity, or systemic toxicity with mucosal application (36). The potential role of NB-401 as an inhaled antimicrobial is supported by preliminary studies showing that multiple daily inhaled doses of undiluted NB-401 (2,000 μg/ml) are well tolerated in mice, with no apparent pulmonary pathology observed upon postmortem examination (our unpublished data). These observations raise the possibility that inhaled NB-401 and hypertonic saline might be useful as a combination inhalational therapy that would have broad-spectrum antimicrobial effects without increasing the already high treatment burden for CF patients. Clearly, however, more comprehensive preclinical efficacy and toxicity testing is required, and these studies are under way. Future studies will assess the pharmacokinetics of NB-401 in CF patients and the concentration of NB-401 achievable within the airway lumen and lung parenchyma following inhalation. Such studies will further characterize the potential utility of NB-401 as an inhaled antimicrobial for the management of CF.
Acknowledgments
This work was funded by the Carroll Haas Research Fund for Cystic Fibrosis and the Michigan Nanotechnology Institute for Medicine and the Biological Sciences. J.L. was supported by funding from the Cystic Fibrosis Foundation. P.E.M. was supported by grant T32 RR07008 from the National Center of Research Resources of the National Institutes of Health. J.R.B. reports a financial interest in NanoBio Corp., which holds a license to commercialize therapies based on nanoemulsion technology.
Footnotes
Published ahead of print on 27 October 2008.
REFERENCES
- 1.Ayres, H. M., J. R. Furr, and A. D. Russell. 1999. Effect of permeabilizers on antibiotic sensitivity of Pseudomonas aeruginosa. Lett. Appl. Microbiol. 28:13-16. [DOI] [PubMed] [Google Scholar]
- 2.Baker, J. R., Jr., and T. Hamouda. January 2008. Nanoemulsion vaccines. U.S. patent 7,314,624.
- 3.Baltimore, R. S., C. D. Christie, and G. J. Smith. 1989. Immunohistopathologic localization of Pseudomonas aeruginosa in lungs from patients with cystic fibrosis. Implications for the pathogenesis of progressive lung deterioration. Am. Rev. Respir. Dis. 140:1650-1661. [DOI] [PubMed] [Google Scholar]
- 4.Bernhardt, S. A., T. Spilker, T. Coffey, and J. J. LiPuma. 2003. Burkholderia cepacia complex in cystic fibrosis: frequency of strain replacement during chronic infection. Clin. Infect. Dis. 37:780-785. [DOI] [PubMed] [Google Scholar]
- 5.Biddick, R., T. Spilker, A. Martin, and J. J. LiPuma. 2003. Evidence of transmission of Burkholderia cepacia, Burkholderia multivorans and Burkholderia dolosa among persons with cystic fibrosis. FEMS Microbiol. Lett. 228:57-62. [DOI] [PubMed] [Google Scholar]
- 6.Bielinska, A. U., K. W. Janczak, J. J. Landers, P. Makidon, L. E. Sower, J. W. Peterson, and J. R. Baker, Jr. 2007. Mucosal immunization with a novel nanoemulsion-based recombinant anthrax protective antigen vaccine protects against Bacillus anthracis spore challenge. Infect. Immun. 75:4020-4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns, J. L. 2007. Antibiotic resistance of Burkholderia spp, p. 81-91. In T. Coenye and P. Vandamme (ed.), Burkholderia: molecular biology and genomics. Horizon Press, Wymondham, United Kingdom.
- 8.Campbell, P. W., III, and L. Saiman. 1999. Use of aerosolized antibiotics in patients with cystic fibrosis. Chest 116:775-788. [DOI] [PubMed] [Google Scholar]
- 9.Caraher, E., G. Reynolds, P. Murphy, S. McClean, and M. Callaghan. 2007. Comparison of antibiotic susceptibility of Burkholderia cepacia complex organisms when grown planktonically or as biofilm in vitro. Eur. J. Clin. Microbiol. Infect. Dis. 26:213-216. [DOI] [PubMed] [Google Scholar]
- 10.Cerca, N., S. Martins, F. Cerca, K. K. Jefferson, G. B. Pier, R. Oliveira, and J. Azeredo. 2005. Comparative assessment of antibiotic susceptibility of coagulase-negative staphylococci in biofilm versus planktonic culture as assessed by bacterial enumeration or rapid XTT colorimetry. J. Antimicrob. Chemother. 56:331-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ceri, H., M. Olson, D. Morck, D. Storey, R. Read, A. Buret, and B. Olson. 2001. The MBEC assay system: multiple equivalent biofilms for antibiotic and biocide susceptibility testing. Methods Enzymol. 337:377-385. [DOI] [PubMed] [Google Scholar]
- 12.Chen, J. S., K. A. Witzmann, T. Spilker, R. J. Fink, and J. J. LiPuma. 2001. Endemicity and inter-city spread of Burkholderia cepacia genomovar III in cystic fibrosis. J. Pediatr. 139:643-649. [DOI] [PubMed] [Google Scholar]
- 13.Chepurnov, A. A., L. F. Bakulina, A. A. Dadaeva, E. N. Ustinova, T. S. Chepurnova, and J. R. Baker, Jr. 2003. Inactivation of Ebola virus with a surfactant nanoemulsion. Acta Trop. 87:315-320. [DOI] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A7, 7th ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 15.Coenye, T., and J. J. LiPuma. 2002. Multilocus restriction typing: a novel tool for studying global epidemiology of Burkholderia cepacia complex infection in cystic fibrosis. J. Infect. Dis. 185:1454-1462. [DOI] [PubMed] [Google Scholar]
- 16.Coenye, T., and J. J. LiPuma. 2002. Population structure analysis of Burkholderia cepacia genomovar III: varying degrees of genetic recombination characterize major clonal complexes. Microbiology 149:77-88. [DOI] [PubMed] [Google Scholar]
- 17.Desai, M., T. Bühler, P. H. Weller, and M. R. Brown. 1998. Increasing resistance of planktonic and biofilm cultures of Burkholderia cepacia to ciprofloxacin and ceftazidime during exponential growth. J. Antimicrob. Chemother. 42:153-160. [DOI] [PubMed] [Google Scholar]
- 18.Donaldson, S. H., W. D. Bennett, K. L. Zeman, M. R. Knowles, R. Tarran, and R. C. Boucher. 2006. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N. Engl. J. Med. 354:241-250. [DOI] [PubMed] [Google Scholar]
- 19.Elkins, M. R., M. Robinson, B. R. Rose, C. Harbour, C. P. Moriarty, G. B. Marks, E. G. Belousova, W. Xuan, and P. T. Bye. 2006. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N. Engl. J. Med. 354:229-240. [DOI] [PubMed] [Google Scholar]
- 20.Favre-Bonté, S., J. C. Pache, J. Robert, D. Blanc, J. C. Pechère, and C. van Delden. 2002. Detection of Pseudomonas aeruginosa cell-to-cell signals in lung tissue of cystic fibrosis patients. Microb. Pathog. 32:143-147. [DOI] [PubMed] [Google Scholar]
- 21.Gibson, R. L., J. Emerson, S. McNamara, J. L. Burns, M. Rosenfeld, A. Yunker, N. Hamblett, F. Accurso, M. Dovey, P. Hiatt, M. W. Konstan, R. Moss, G. Retsch-Bogart, J. Wagener, D. Waltz, R. Wilmott, P. L. Zeitlin, and B. Ramsey. 2003. Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 167:841-849. [DOI] [PubMed] [Google Scholar]
- 22.Grebski, E., C. Peterson, and T. C. Medici. 2001. Effect of physical and chemical methods of homogenization on inflammatory mediators in sputum of asthma patients. Chest 119:1521-1525. [DOI] [PubMed] [Google Scholar]
- 23.Hamouda, T., and J. R. Baker, Jr. 2000. Antimicrobial mechanism of action of surfactant lipid preparations in enteric gram-negative bacilli. J. Appl. Microbiol. 89:397-403. [DOI] [PubMed] [Google Scholar]
- 24.Hamouda, T., M. M. Hayes, Z. Cao, R. Tonda, K. Johnson, D. C. Wright, J. Brisker, and J. R. Baker, Jr. 1999. A novel surfactant nanoemulsion with broad-spectrum sporicidal activity against Bacillus species. J. Infect. Dis. 180:1939-1949. [DOI] [PubMed] [Google Scholar]
- 25.Hamouda, T., A. Myc, B. Donovan, A. Y. Shih, J. D. Reuter, and J. R. Baker, Jr. 2001. A novel surfactant nanoemulsion with a unique non-irritant topical antimicrobial activity against bacteria, enveloped viruses and fungi. Microbiol. Res. 156:1-7. [DOI] [PubMed] [Google Scholar]
- 26.Hart, J. R. 1984. Chelating agents as preservative potentiators, p. 54-58. In J. J. Kabara (ed.), Cosmetic and drug preservation: principles and practices. Marcell Dekker, New York, NY.
- 27.Hasan, M. A., and C. F. Lange. 2007. Estimating in vivo airway surface liquid concentration in trials of inhaled antibiotics. J. Aerosol Med. 20:282-293. [DOI] [PubMed] [Google Scholar]
- 28.Hunt, B. E., A. Weber, A. Berger, B. Ramsey, and A. L. Smith. 1995. Macromolecular mechanisms of sputum inhibition of tobramycin activity. Antimicrob. Agents Chemother. 39:34-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson, W. M., S. D. Tyler, and K. R. Rozee. 1994. Linkage analysis of geographic and clinical clusters in Pseudomonas cepacia infections by multilocus enzyme electrophoresis and ribotyping. J. Clin. Microbiol. 32:924-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones, T., M. Flack, M. M. Ijzerman, and J. R. Baker, Jr. 2008. Safety, tolerance, and pharmacokinetics of topical nanoemulsion (NB-002) for the treatment of onychomycosis. J. Am. Acad. Dermatol. 58(Suppl. 2):AB83. [Google Scholar]
- 31.Jones, T., M. Flack, L. Stanberry, and J. R. Baker, Jr. 2008. Safety, tolerance, pharmacokinetics, and efficacy of topical nanoemulsion (NB-001) for the treatment of herpes labialis. J. Am. Acad. Dermatol. 58(Suppl. 2):AB93. [Google Scholar]
- 32.Lambert, R. J., G. W. Hanlon, and S. P. Denyer. 2004. The synergistic effect of EDTA/antimicrobial combinations on Pseudomonas aeruginosa. J. Appl. Microbiol. 96:244-253. [DOI] [PubMed] [Google Scholar]
- 33.Lewin, C., C. Doherty, and J. Govan. 1993. In vitro activities of meropenem, PD 127391, PD 131628, ceftazidime, chloramphenicol, co-trimoxazole, and ciprofloxacin against Pseudomonas cepacia. Antimicrob. Agents Chemother. 37:123-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LiPuma, J. J. 2001. Burkholderia cepacia complex: a contraindication to lung transplantation in cystic fibrosis? Transpl. Infect. Dis. 3:149-160. [DOI] [PubMed] [Google Scholar]
- 35.LiPuma, J. J. 2005. Update on the Burkholderia cepacia complex. Curr. Opin. Pulm. Med. 11:528-533. [DOI] [PubMed] [Google Scholar]
- 36.Makidon, P. E., A. U. Bielinska, S. S. Nigavekar, K. W. Janczak, J. Knowlton, A. J. Scott, N. Mank, Z. Cao, S. Rathinavelu, M. R. Beer, J. E. Wilkinson, L. P. Blanco, J. J. Landers, and J. R. Baker, Jr. 2008. Pre-clinical evaluation of a novel nanoemulsion-based hepatitis B mucosal vaccine. PLoS ONE 3:e2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Möller, L. V., W. Timens, W. van der Bij, K. Kooi, B. de Wever, J. Dankert, and L. van Alphen. 1998. Haemophilus influenzae in lung explants of patients with end-stage pulmonary disease. Am. J. Respir. Crit. Care Med. 157:950-956. [DOI] [PubMed] [Google Scholar]
- 38.Moore, R. A., and R. E. Hancock. 1986. Involvement of outer membrane of Pseudomonas cepacia in aminoglycoside and polymyxin resistance. Antimicrob. Agents Chemother. 30:923-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moreau-Marquis, S., B. A. Stanton, and G. A. O'Toole. 2008. Pseudomonas aeruginosa biofilm formation in the cystic fibrosis airway. Pulm. Pharmacol. Ther. 21:595-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moriarty, T. F., J. C. McElnay, J. S. Elborn, and M. M. Tunney. 2007. Sputum antibiotic concentrations: implications for treatment of cystic fibrosis lung infection. Pediatr. Pulmonol. 42:1008-1017. [DOI] [PubMed] [Google Scholar]
- 41.Moskowitz, S. M., J. M. Foster, J. Emerson, and J. L. Burns. 2004. Clinically feasible biofilm susceptibility assay for isolates of Pseudomonas aeruginosa from patients with cystic fibrosis. J. Clin. Microbiol. 42:1915-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myc, A., J. F. Kukowska-Latallo, A. U. Bielinska, P. Cao, P. P. Myc, K. Janczak, T. R. Sturm, M. S. Grabinski, J. J. Landers, K. S. Young, J. Chang, T. Hamouda, M. A. Olszewski, and J. R. Baker, Jr. 2003. Development of immune response that protects mice from viral pneumonitis after a single intranasal immunization with influenza A virus and nanoemulsion. Vaccine 21:3801-3814. [DOI] [PubMed] [Google Scholar]
- 43.Myc, A., T. Vanhecke, J. J. Landers, T. Hamouda, and J. R. Baker, Jr. 2001. The fungicidal activity of novel nanoemulsion (X8W60PC) against clinically important yeast and filamentous fungi. Mycopathologia 155:195-201. [DOI] [PubMed] [Google Scholar]
- 44.Peeters, E., H. J. Nelis, and T. Coenye. 2008. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 72:157-165. [DOI] [PubMed] [Google Scholar]
- 45.Peeters, E., H. J. Nelis, and T. Coenye. 2008. Resistance of planktonic and biofilm-grown Burkholderia cepacia complex isolates to the transition metal gallium. J. Antimicrob. Chemother. 61:1062-1065. [DOI] [PubMed] [Google Scholar]
- 46.Pettit, R. K., C. A. Weber, M. J. Kean, H. Hoffmann, G. R. Pettit, R. Tan, K. S. Franks, and M. L. Horton. 2005. Microplate Alamar blue assay for Staphylococcus epidermidis biofilm susceptibility testing. Antimicrob. Agents Chemother. 49:2612-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pope, C. F., S. H. Gillespie, J. R. Pratten, and T. D. McHugh. 2008. Fluoroquinolone-resistant mutants of Burkholderia cepacia. Antimicrob. Agents Chemother. 52:1201-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reik, R., T. Spilker, and J. J. LiPuma. 2005. Distribution of Burkholderia cepacia complex species among isolates recovered from persons with or without cystic fibrosis. J. Clin. Microbiol. 43:2926-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sajjan, U., M. Corey, A. Humar, E. Tullis, E. Cutz, C. Ackerley, and J. Forstner. 2001. Immunolocalisation of Burkholderia cepacia in the lungs of cystic fibrosis patients. J. Med. Microbiol. 50:535-546. [DOI] [PubMed] [Google Scholar]
- 50.Schwab, U., M. Leigh, C. Ribeiro, J. Yankaskas, K. Burns, P. Gilligan, P. Sokol, and R. Boucher. 2002. Patterns of epithelial cell invasion by different species of the Burkholderia cepacia complex in well-differentiated human airway epithelia. Infect. Immun. 70:4547-4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh, P. K., A. L. Schaefer, M. R. Parsek, T. O. Moninger, M. J. Welsh, and E. P. Greenberg. 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762-764. [DOI] [PubMed] [Google Scholar]
- 52.Smith, A. L., G. Redding, C. Doershuk, D. Goldmann, E. Gore, B. Hilman, M. Marks, R. Moss, B. Ramsey, T. Roblo, R. H. Schwartz, M. J. Thomassen, J. Williams-Warner, A. Weber, R. W. Wilmott, H. D. Wilson, and R. Yogev. 1988. Sputum changes associated with therapy for endobronchial exacerbation in cystic fibrosis. J. Pediatr. 112:547-554. [DOI] [PubMed] [Google Scholar]
- 53.Stepanović, S., D. Vuković, I. Dakić, B. Savić, and M. Švabić-Vlahović. 2000. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 40:175-179. [DOI] [PubMed] [Google Scholar]
- 54.Stewart, P. S., and J. W. Costerton. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135-138. [DOI] [PubMed] [Google Scholar]
- 55.Taccetti, G., S. Campana, L. Marianelli, et al. 1999. Multiresistant non-fermentative gram-negative bacteria in cystic fibrosis patients: the results of an Italian multicenter study. Eur. J. Epidemiol. 15:85-88. [DOI] [PubMed] [Google Scholar]
- 56.Taneja, N. K., and J. S. Tyagi. 2007. Resazurin reduction assays for screening of anti-tubercular compounds against dormant and actively growing Mycobacterium tuberculosis, Mycobacterium bovis BCG and Mycobacterium smegmatis. J. Antimicrob. Chemother. 60:288-293. [DOI] [PubMed] [Google Scholar]
- 57.Tré-Hardy, M., C. Macé, N. El Manssouri, F. Vanderbist, H. Traore, and M. J. Devleeschouwer. Effect of antibiotic co-administration on young and mature biofilms of cystic fibrosis clinical isolates: the importance of the biofilm model. Int. J. Antimicrob. Agents, in press. [DOI] [PubMed]
- 58.Ulrich, M., S. Herbert, J. Berger, G. Bellon, D. Louis, G. Munker, and G. Doring. 1998. Localization of Staphylococcus aureus in infected airways of patients with cystic fibrosis and in a cell culture model of S. aureus adherence. Am. J. Respir. Cell Mol. Biol. 19:83-91. [DOI] [PubMed] [Google Scholar]
- 59.Vaara, M. 1992. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 56:395-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vandamme, P., B. Holmes, M. Vancanneyt, T. Coenye, B. Hoste, R. Coopman, H. Revets, S. Lauwers, M. Gillis, K. Kersters, and J. R. Govan. 1997. Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and proposal of Burkholderia multivorans sp. nov. Int. J. Syst. Bacteriol. 47:1188-1200. [DOI] [PubMed] [Google Scholar]
- 61.Weber, D. J., W. A. Rutala, and E. E. Sickbert-Bennett. 2007. Outbreaks associated with contaminated antiseptics and disinfectants. Antimicrob. Agents Chemother. 51:4217-4224. [DOI] [PMC free article] [PubMed] [Google Scholar]