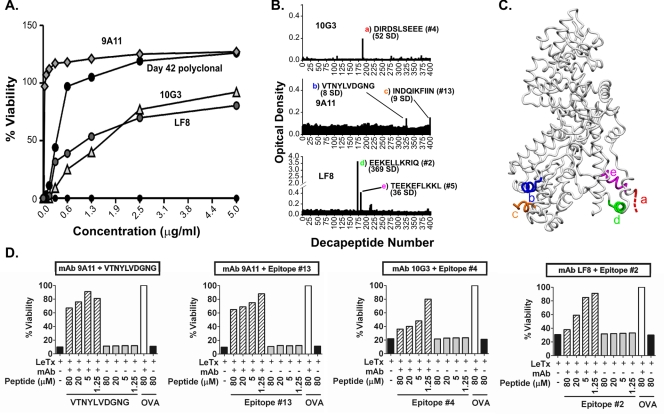
FIG. 4.
Neutralizing LF-specific MAbs bind sequential B-cell epitopes of LF. (A) Anti-LF MAbs 10G3, 9A11, and LF8 (27, 41, 51) neutralize anthrax LeTx in vitro. (B) Epitope mapping of LF-specific, LeTx-neutralizing MAbs 10G3, 9A11, and LF8. The sequences of bound decapeptides, the numbers of SD above the background level, and the corresponding epitopes shown in Table 1 are indicated. The colored letters correspond to the colored letters in panel C. (C) Structural representation of five epitopes bound by neutralizing LF-specific MAbs. The colored letters correspond to the colored letters in panel B. Two residues of epitope c are not resolved in the LF crystal structure and thus are not shown. (D) Linear peptides corresponding to MAbs 10G3, 9A11, and LF8 mediate measurable reductions in neutralization in vitro (striped bars). Inhibition of neutralization is compared to the results for the same MAb mixed with toxin and irrelevant peptide from ovalbumin (OVA) (open bars). All experiments were carried out in duplicate. Since duplicate values were nearly identical, error bars are not shown. The concentrations of MAbs used for peptide inhibition (see Materials and Methods) were 1.25 μg/ml for MAb 9A11 and 5 μg/ml for MAbs 10G3 and LF8.