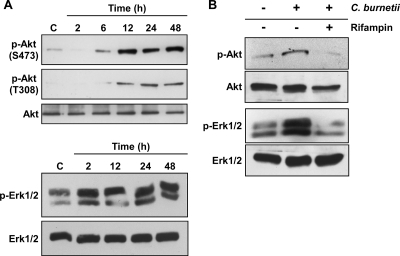
FIG. 4.
Sustained C. burnetii activation of Akt and Erk1/2 is dependent on bacterial protein synthesis. Lysates were harvested from mock- and C. burnetii phase II-infected THP-1 cells at the indicated times postinfection and subjected to immunoblot analysis. In each panel, a representative immunoblot is shown for each protein. Mock-infected THP-1 cell lysates were used as a negative control (lanes C). (A) Akt and Erk1/2 were phosphorylated early after infection and remained activated through 48 hpi. (B) THP-1 cells were infected for 48 h in the presence or absence of rifampin to inhibit C. burnetii RNA synthesis. Cell lysates from mock- and C. burnetii-infected THP-1 cells were subsequently analyzed for phosphorylated Akt and Erk1/2 by immunoblotting. Phosphorylation of each protein was substantially reduced in rifampin-treated, infected cells. p, phosphorylated.