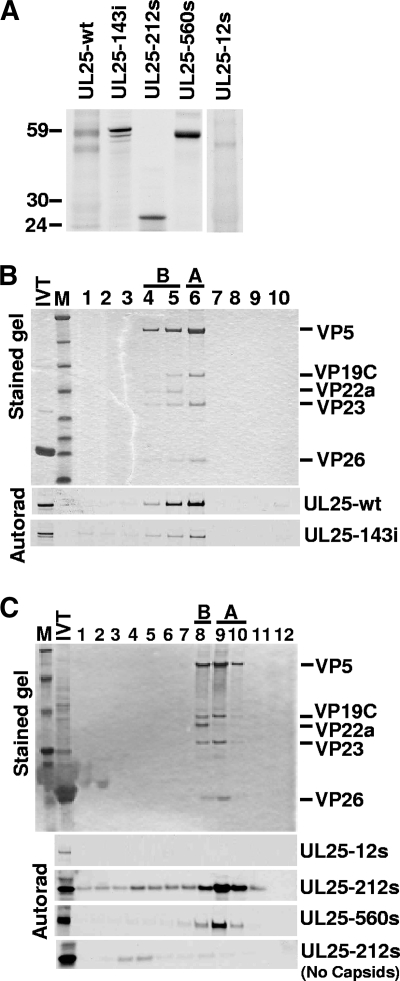
FIG. 3.
Capsid binding by UL25 transposon mutants. (A) Autoradiograph of [35S]Met-labeled UL25 protein after SDS-PAGE. The sizes of the 143i, 212s, and 560s proteins were consistent with predicted molecular mass for each protein, but the 12s mutant unexpectedly produced a nearly wild-type(wt)-size UL25 protein. (B and C) In vitro capsid-binding assay. In vitro-translated protein was incubated with pooled A and B capsids isolated from Vero cells infected with the UL25-null virus, vΔUL25, and capsids were then purified by sucrose gradient centrifugation. The sucrose gradients were fractionated and analyzed by SDS-PAGE followed by Coomassie staining (top panel, representative gel) and autoradiography (bottom panels). The fractions containing A and B capsids are indicated. The capsid proteins visible in the stained gel are listed at right. Capsid binding was demonstrated by comigration of the UL25 protein with A and B capsids. Aggregation of the truncated 212s protein may explain the presence of this protein in the same dense fractions as in the gradient lacking A and B capsids (bottom of panel C). IVT, in vitro transcription-translation; M, molecular mass markers (from top, 194, 104, 60, 41, 27, 21, 16, and 7 kDa). Lane 1 is the bottom of the gradient and lane 10 (B) or 12 (C) is the top.