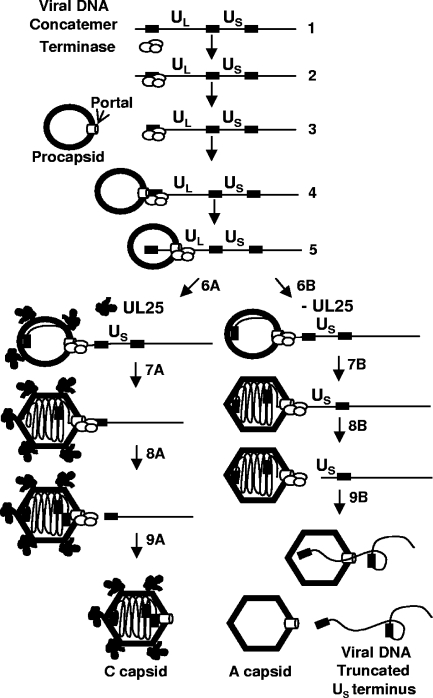
FIG. 8.
Model for the role of UL25 in DNA packaging. Proposed model for the role of UL25 in HSV DNA encapsidation. (Step 1) DNA packaging initiates on concatemers when the terminase complex consisting of UL15, UL28, and UL33 binds (step 2) to packaging sequences and cleaves (step 3) the concatemer to generate a UL end with the terminase still bound. The terminase-DNA complex then docks at the portal vertex on the procapsid (step 4), and the terminase initiates DNA packaging (step 5). As the procapsid is filled (step 6A) with DNA, it angularizes and in the process, UL25 binding sites are exposed at the capsid vertexes. DNA cleavage is suppressed by a headful mechanism (step 7A) until a full-length genome has entered. Cleavage occurs at a packaging site located at the US end of the genome (step 8A). More UL25 binds to the capsid, and the newly packaged genome is sealed within the C capsid (step 9A). In the absence of a functional UL25 protein, the packaging reaction proceeds (step 6B) with the capsid filling and expanding until close to a genome length has entered (step 7B). Without UL25 to stabilize the capsid against the pressure that the packaged genome generates, premature cleavage (step 8B) occurs just prior to the entry of the US-end repeat, resulting in genomes with truncated US termini. In the absence of UL25, the capsid is not stable. The DNA is released (step 9B), which generates an empty A capsid and a free viral genome that is truncated at the US end.