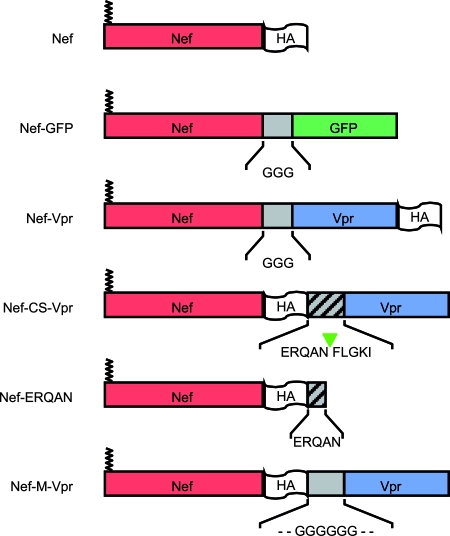
FIG. 1.
Nef-based constructs used in this study. The various domains of the chimeric proteins are illustrated. Except for Nef-GFP, all constructs are HA tagged to facilitate the immunodetection in Western blotting experiments. The HA tag was added to the C-terminal ends of the proteins in order to preserve the myristoylation site of Nef. In the case of Nef-CS-Vpr, an HIV-1 protease recognition motif corresponding to the NC-p1 junction in the Gag-Pol precursor was inserted between Nef and Vpr. The HA tag was moved between Nef and this motif in order to visualize Nef fragments generated from the cleavage of the fusion protein in the virus. A similar construct was also generated that contains a glycine stretch in place of the protease recognition motif. Because the processing of Nef-CS-Vpr is supposed to release Nef fragments with the ERQAN sequence C terminal to the HA tag, a Nef-ERQAN was also generated and analyzed in incorporation and infectivity experiments.