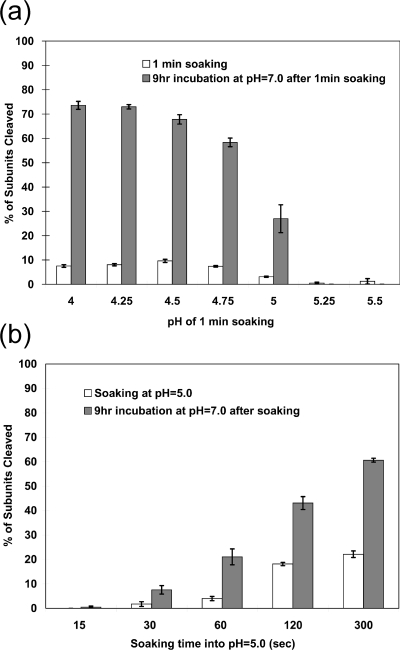
FIG. 4.
Autoproteolysis occurs at neutral pH after activation at low pH. (a) The progress of the autoproteolytic cleavage at neutral pH values following activation at lower pH. The wild-type VLP procapsids were soaked in buffers between pH 4.00 and 5.50 for 1.0 min. The pH was then immediately shifted to pH 7.0, and the particles were incubated for 9.0 h. The fractions of subunits cleaved at 1 min and 9 h were determined as described for Fig. 3. (b) The progress of the autoproteolytic cleavage at neutral pH values following incubation at pH 5.0 for between 15 and 300 s. After being soaked at the indicated time points, the pH values were immediately changed to pH 7.0 and the mixture was incubated for 9 h. The chemistry of the reaction does not require low pH. The data indicate that ∼10% of the subunits must initially cleave at low pH for the reaction to proceed at pH 7.0, a result consistent with the observations of Canady et al. (7). We conclude that when fewer subunits cleave, the LCC is reversible. Panel b shows a relationship between the amount of initial cleavage and that seen after the reaction proceeds, suggesting that acidic residues with pKa values above 5.0 would play a key role in the LCC at low pH values.