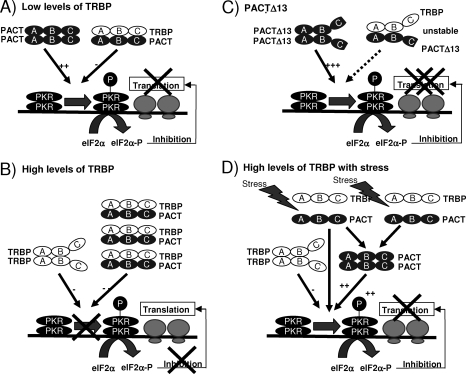
FIG. 10.
Model for the controlled activation of PKR by TRBP, PACT, and stress. (A) PKR activation in cells with low levels of TRBP. In cells where TRBP concentration is low, PACT can activate PKR, which in turn phosphorylates eIF2α (eIF2α-P). eIF2α-P blocks viral and cellular translation initiation. (B) Absence of PKR activation in cells with high levels of TRBP. When TRBP concentration is high, PACT forms heterodimers with TRBP that inhibit PKR phosphorylation (P) and overcome its translation block. PACT is unable to activate PKR and, therefore, mRNA translation remains active. (C) Overexpression of PACTΔ13 strongly activates PKR. When PACTΔ13 is overexpressed in cells, the heterodimer with TRBP is unstable. As a consequence, PACTΔ13 strongly activates PKR. P-PKR has a decreased affinity for PACTΔ13 and becomes available to activate eIF2α, which blocks translation initiation. (D) Stress treatment dissociates TRBP-PACT interaction and activates PKR. A stress treatment induces a separation between TRBP and PACT. Therefore, PACT becomes available for PKR activation either as a monomer or as a dimer. In all panels, A, B, and C represent regions that encompass dsRBD1, dsRBD2, and the Medipal domains, respectively.