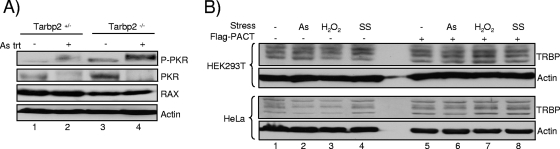
FIG. 8.
Stresses activate PKR but do not modify TRBP levels significantly. (A) Stress mediated by arsenite treatment induces higher PKR phosphorylation in Tarbp2−/− cells. Tarbp2+/− and Tarbp2−/− TEFs were either untreated (lanes 1 and 3) or treated with 0.5 mM sodium arsenite (As) for 1 h (lanes 2 and 4). Whole-cell extracts (200 μg) were subjected to SDS-PAGE and blotted with anti-P-PKR, anti-PKR, anti-PACTeg2, and antiactin antibodies as indicated. RAX, the murine homolog of PACT, was detected with the anti-PACT. The lower-migrating band revealed with anti-P-PKR in the absence of sodium arsenite treatment (lanes 1 and 3) corresponds to the size of PKR shown just below and might represent a different phosphorylated form in murine cells. (B) Stresses do not modify TRBP levels significantly. HEK293T cells were transfected without plasmid (lanes 1 to 4) or with 2 μg of pCMV2-Flag-PACT (lanes 5 to 8) and not treated (lanes 1 and 5) or treated with 0.5 mM sodium arsenite (As) for 1 h (lanes 2 and 6) or 10 mM hydrogen peroxide (H2O2) for 1 h or subjected to serum starvation (SS) for 24 h. A total of 150 μg of proteins from each lysate was run on a 10% SDS-PAGE gel and blotted using anti-TRBPjbx and antiactin antibodies.