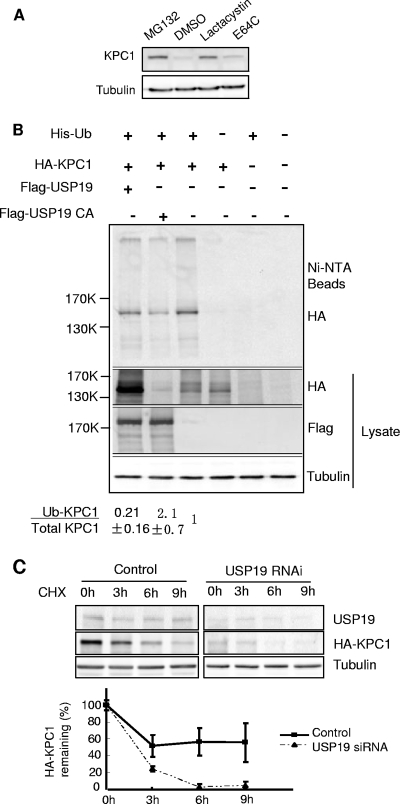
FIG. 4.
KPC1 is a substrate of the ubiquitin-proteasome pathway and is stabilized by USP19. (A) KPC1 is stabilized by proteasome inhibitors. FR3T3 cells were treated with a proteasome inhibitor, either MG132 (10 μΜ) or lactacystin (5 μΜ), or with cysteine protease inhibitor E64C (10 μΜ) or vehicle (DMSO [dimethyl sulfoxide]) for 6 h. Cells were then harvested and blotted with anti-KPC1 antibody. (B) KPC1 is polyubiquitinated in vivo, and the ubiquitination is modulated by USP19. FR3T3 cells were transfected with the indicated plasmids. Cells were treated with MG132 for 6 h before lysis to accumulate ubiquitinated proteins. Lysate was either analyzed by immunoblotting with the indicated antibodies or incubated with Ni+-agarose beads. Bound ubiquitinated proteins were washed extensively and then eluted with Laemmli buffer and analyzed by immunoblotting with anti-HA antibody. Quantitation and ratios of the levels of ubiquitinated KPC1 (anti-HA blot of proteins bound to Ni+-NTA beads) to total KPC1 (anti-HA blot of lysate) (means ± standard errors) are indicated below. Means for cells overexpressing wild-type USP19 or the USP19CA mutant are significantly different (P value of <0.025 by one-way analysis of variance) from that for non-USP19-overexpressing cells, which was set at 1. Ub, ubiquitin; +, present; −, absent. (C) Silencing of USP19 destabilizes KPC1. FR3T3 cells stably overexpressing KPC1 were transfected with USP19 siRNA or control oligonucleotides. Forty-eight hours later, the cells were incubated with cycloheximide. At the indicated times, cell protein was analyzed by immunoblotting with the indicated antibodies. Shown are representative immunoblots and quantitation of KPC1 levels from triplicate samples. Error bars show standard errors. CHX, cycloheximide; RNAi, RNA interference.