Abstract
Changes in selected blood and serum components and electrocardiography (ECG) were investigated in 20 adults (13 females and 7 males) of water buffaloes suffering from severe theileriosis. The age of all animals used in this study ranged 1.5-5 yr. Theileriosis was diagnosed by observation of parasites in the peripheral blood and the presence of schizonts in lymphocytes that were provided from swollen lymph nodes. Statistically significant decreases were observed in the means of RBC, WBC, and packed cell volume (PCV) in blood of infected animals. The means levels of sodium, calcium, phosphorus, and potassium of infected animals were lower than healthy animals, but only the decrease of potassium was significant. The mean serum activities of aspartate transferase and alanine aminotransfrase were significantly higher than in uninfected animals. Three cases had atrial premature beat, 2 cases had sinus tachycardia, 2 had sinus arrhythmia, and 1 had first degree of atrioventricular block in ECG. The present study showed that T. annulata infection in cattle is associated with hematological and biochemical, and ECG changes.
Keywords: Theileria annulata, biochemical parameter, hematological parameter, buffalo, theileriosis
INTRODUCTION
Tropical theileriosis, a tick-borne hemoprotozoan disease caused by Theileria annulata and transmitted by Hyalomma spp., is one of the most devastating blood parasites affecting cattle, buffalo, and sheep. The prevalence, morbidity, and mortality of tropical theileriosis are considerably high [1,2]. It is estimated that 250 million cattle in many countries including Iran, Turkey, India, and China are at a risk of the disease, which causes serious economic loss through bovine mortality and loses productivity [3,4]. In their mammalian hosts, the parasites have a complex life cycle. Infection is initiated by the transformation of macroschizont-infected cells in the lymph nodes draining the site of inoculation of sporozoites by ticks. The cattle infective form of the parasite is the sporozoite transforming into schizonts in WBC of the mononuclear lineage. The schizont undergoes further differentiation to merozoites, which are released upon lysis of the infected cells. Once released from host cells, the merozoites enter erythrocytes. This is followed by the development of piroplasms in erythrocytes and the parasite becomes infective for the vector [5,6].
Weakness, weight loss, anorexia, high body temperature, petechia on the conjunctival mucosa, swollen lymph nodes, anemia, and cough are the most common clinical symptoms in theileriosis. On later stages of theileriosis, infected animals cannot stand up, their body temperatures are under normal values (< 38.5℃), and ichterus, dehydration, and blood in feces are occasional clinical symptoms [7,8]. Biochemical and hematological parameters and ECG may be changed in this disease [4,8]. The present study was conducted on 20 affected buffaloes for the determination of these changes.
MATERIALS AND METHODS
Animals and samples for clinical investigations
This research was carried out during the disease season from June to October 2007 in Tabriz, Iran. Twenty adults (13 females and 7 males) of water buffaloes with severe theileriosis and 20 healthy buffaloes were selected. The age of all animals used in this study ranged 1.5-5 yr. Infected buffaloes were diagnosed by clinical signs and observation of parasites in the blood (which are prepared from the auricular vein) and lymph node samples. The animals showed a high percentage of parasitemia. The parasites were evident as schizonts, sometimes in circulating lymphocytes, but mainly in biopsy smears of enlarged lymph nodes stained with Giemsa. Piroplasms were also easily visible in erythrocytes. Over 20-30% of RBC are infected. Blood-sucking ticks were found on many parts of the buffaloes and were identified as Hyalomma spp. The clinical manifestations were enlargement of the prescapular lymph nodes, pyrexia, inappetence, cachexia, mucous membrane discharge, hemorrhages, dyspnea, cessation of rumination, protrusion of the eyeball, lacrimation, and conjunctivitis.
The smears were air-dried, fixed with methanol, stained with Giemsa, and carefully examined under the oil immersion objective of a microscope to estimate the degree of infection. For estimating parasitemia, the percentage of piroplasm-infected erythrocytes was calculated among 100 cells. Similarly, smears of lymph node biopsies were stained with Giemsa and examined for schizonts. Clinical and parasitological observations were recorded for all the animals showing the clinical signs of T. annulata infection. Two samples of blood were obtained from the jugular vein of buffaloes. The sample with anticoagulant was used to do hematological tests and to measure PCV, RBC, WBC, and differential count of WBC. The RBC and WBC counts were determined by a hemocytometer. The other sample was centrifuged to take the sera, and the biochemical parameters such as total protein, calcium, phosphorus, alanine aminotransferase (ALT), aspartate aminotransferase (ALT), sodium and potassium levels of sera were measured. The PCV % was measured by microcentrifuge. The levels of total protein, calcium, phosphorus, alkaline phosphatase, and aspartate aminotransferase were measured with biochemical methods, and those of sodium and potassium with a flame photometer [9-11].
ECG
After 30 min rest, ECG using 3 channel ECG system and base apex lead was obtained in about 30 sec. The positive electrode of lead I was attached to the skin of the left thorax at the 5th intercostal space immediately caudal to the olecranon, and the negative electrode placed on the jugular furrow in the caudal third of the right neck. The ground electrode was placed remote from the heart. The electrodes were placed using alligator clips and gel. In order to ensure good adherence of electrodes to the skin, that coat of the skin was shaved and cleaned with alcohol prior to the application of gel [8]. Types of arrhythmias were determined.
Statistical analysis
The differences of means of laboratory values between buffaloes with theileriosis and normal animals were compared using the Student's t-test. The presence of cardiac arrhythmias was analyzed as percent. Values of P < 0.05 or P < 0.01 were considered significant.
RESULTS
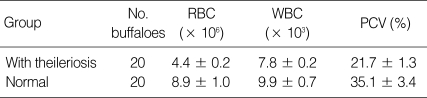
In hematological analyses, decreases were observed in the mean RBC count, hematocrit value, hemoglobin amount, and WBC count in buffaloes with thileriosis. The mean of RBC was 4.4 ± 0.2 × 106 and WBC was 7.8 ± 0.2 × 103 in these animals. The mean % of PCV in the sick buffaloes was 21.7 ± 1.3% (Table 1).
Table 1.
Mean levels of hematological parameters in the buffaloes with theilleriosis and normal
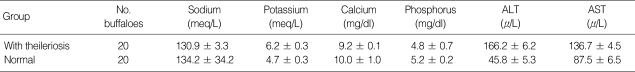
The serum levels of sodium and potassium in the buffaloes with theileriosis were 130.9 ± 3.3 and 6.2 ± 0.3 meq/L, respectively. Calcium and phosphorus mean levels were 9.2 ± 0.1 and 4.8 ± 0.7 mg/dl, respectively, and the mean level of total protein in the serum was 8.5 ± 0.1 gr/dl. The serum levels of ALT and AST enzymes were 166.2 ± 6.2 and 136.7 ± 4.5 µ/L, respectively (Table 2).
Table 2.
Mean levels of sodium, potassium, calcium, and phosphorus, and ALT and AST enzymes in sera of the buffaloes with theileriosis and normal
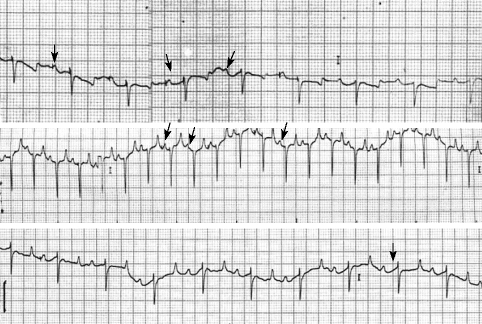
In theileriosis buffaloes, 3 cases had atrial premature beat, 2 cases had sinus tachycardia, 2 had sinus arrhythmia, and 1 had first degree of atrioventricular block, while normal animals had any ECG changes (Fig. 1).
Fig. 1.
Kinds of cardiac arrhythmias in the buffaloes with theileriosis. A, normal; B, atrial premature beat; C, sinus arrhythmia; D, first degree of atrioventricular block.
DISCUSSION
T. annulata is a tick-borne protozoan parasite of cattle and buffaloes that infects leukocytes and causes an acute, lymphoproliferative disease known as tropical theileriosis. Tropical theileriosis has a particularly devastating impact on small-holder farmers, which represent the majority of livestock owners in endemic areas. The methods currently used to protect against tropical theileriosis are expensive and all have serious limitations in efficacy and sustainability. Therefore, there is a need and demand for improved control methods focused on the disease prevention [4,12,13].
In the present study, animals showed a high percentage of parasitemia and exhibiting clinical signs of tropical theileriosis. T. annulata spreads through the lymphoid system and other organs rapidly, and induces production of TNF-α and IFN-γ. These cytokines disrupt the physiological integrity of the host. Moreover, the presence of parasites in the pituitary and adrenal glands can cause disturbance of the immune and endocrine systems [14,15]. Cytokines (TNF-α, IL-1, and IL-6) produced by infected mononuclear cells are responsible for the diverse clinical symptoms of tropical theileriosis, such as depression, pyrexia, anorexia, cachexia, and disseminated hemorrhages [16].
RBC counts and hematocrit values were lower in infected animals compared to normal ranges. These results were in agreement with other reports [2,17]. Erythrophagocytosis due to an immune-mediated mechanism might be responsible for the erythrocyte destruction [18]. Removal of the piroplasm-infected erythrocytes by macrophages in the organs of the reticuloendothelial system has been suggested as a cause of anemia [17, 19]. In addition, pro-inflammatory cytokines, particularly TNF-α, have been implicated in mediating anemia associated with tropical theileriosis [14,16]. The decrease in RBC could be due to increased levels of activated complement products. Additionally, since oxidized erythrocytes may be destroyed easily by erythropagocytosis, oxygen radicals may also be involved in the pathogenesis of anemia [20-22].
In this study, leucopenia was seen in infected animals. Some researchers have demonstrated that the leucocyte count increased immediately following Theileria infection and then significantly decreased within several days [23,24]. T. annulata-induced leucopoenia is mainly mediated by TNF-α [14]. This decrease is related to a destruction of lymphocytes in lymphoid organs and infiltration of these cells into various organs [2,24].
In our study, the buffaloes with theileriosis had hyponatremia, hypocalcemia, hypophosphatemia, and hyperkalemia. The difference between the means of sodium, calcium, and phosphorus in the sera of animals with theileriosis and normal ranges in healthy animals were not significant, but the levels of potassium were significantly (P < 0.05) different. In theileriosis, hypocalcaemia and hyponatremia were probably due to the hypoproteinemia, decreased dietary intake, intestinal malfunction, and kidney damage. The decreased serum phosphorus concentration in cattle with theileriosis is the results of diarrhea and renal wasting. The AST and ALT values in animals with theileriosis were significantly higher than the normal values (P < 0.01). These finding supports the results of Hilali et al. [19] and Singh et al. [25] obtained in their studies on theileriosis. The occurrence of parasites in any tissue causes the parasitic tissue damage. In hepatic injury seen in theileriosis, increased serum activities of AST and ALT are closely associated with the hepatic function. Furthermore, the significant rises in the serum AST and ALT activities were due to muscle trauma caused by prolonged recumbency in theileriosis.
Three cases had atrial premature beat, 2 cases had sinus tachycardia, 2 cases had sinus arrhythmia, and 1 had first degree of atrioventricular block. Variation in the rhythm and rate of the heart in animals can occur due to strong and varying autonomic influence but can also be a reflection of a primary myocardial disease. Other factors such as acid-base imbalance can influence the rate and rhythm. These factors must be taken into considerations in the assessment of apparent abnormalities detected on clinical examination of the cardiovascular system. Sinus tachycardia caused by pain, hyperthermia, anemia, electrolyte imbalance, and fall in the arterial blood pressure [8]. In these buffaloes, there were hyperthermia, anemia, and electrolyte imbalance. In sick cattle and buffaloes, atrial fibrillation most commonly occurs in association with gastrointestinal, metabolic, and hemolytic diseases [8].
In our study buffaloes with theileriosis had anemia, and metabolic and electrolyte imbalances that caused atrial fibrillations. Sinus arrhythmia is a normal physiological arrhythmia that occurs at slow resting heart rates and is associated with variations in the rate of discharge from the sinoatrial node. This arrhythmia also can be induced with electrolyte imbalance. The first degree of atrioventricular block is an ECG diagnosis and cannot be detected clinically. It occurs when conduction is delayed at the atrioventricular node. It can be associated with electrolyte imbalance, overdosing with calcium, digoxin, and cardiomyopathy. In buffaloes with theileriosis maybe there were these factors [8]. ECG changes in buffaloes have not established in other studies, but in cows and horses these had been established. A large scale cross-sectional study of 952 healthy dairy cattle aged 1 or more yr produced the following prevalence of arrhythmias; 8.5% sinus arrhythmia, 1.6% first-degree atrioventricular block, 0.6% ventricular premature complex, 0.4% atrial premature complex, 0.2% sinus bradycardia, and 0.1% ventricular escape beats. Atrial fibrillation was not observed in this study [26]. In a previous study, the efficacy of ketamine and bupivacaine in enhancing the epidural analgesia induced by medetomidine was evaluated in 10 buffalo calves. The ECG changes included tall T wave, QS pattern, RS pattern, ST elevation, and heart blocks at different intervals, which were more frequent and pronounced in animals given bupivacaine with medetomidine [27]. Perinodal myocardial fibrosis and microvascular abnormality have been reported in horse with sinoatrial and atrioventricular block, and considered as the excitatory cause, because myocardial fibrosis is common in horses [28].
According to the kinds of arrhythmias and electrolyte imbalance, it is concluded that arrhythmias in these buffaloes may be physiologic, and treatment as well as balancing of electrolytes will remove arrhythmias. Based on these observations, it can be concluded that severe T. annulata infection is associated with profound changes in hematological and biochemical profiles and ECG parameters.
References
- 1.Aktas M, Dumanli N, Angin M. Cattle infestation by Hyalomma ticks and prevalence of Theileria in Hyalomma species in the east of Turkey. Vet Parasitol. 2004;119:1–8. doi: 10.1016/j.vetpar.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Omer OH, El-Malik KH, Mahmoud OM, Haroun EM, Hawas A, Sweeney D, Magzoub M. Haematological profiles in pure bred cattle naturally infected with Theileria annulata in Saudi Arabia. Vet Parasitol. 2002;107:161–168. doi: 10.1016/s0304-4017(02)00094-8. [DOI] [PubMed] [Google Scholar]
- 3.Campbell JDM, Spooner RL. Macrophages behaving badly: infected cells and subversion of immune responses to Theileria annulata. Parasitol Today. 1999;15:10–16. doi: 10.1016/s0169-4758(98)01359-3. [DOI] [PubMed] [Google Scholar]
- 4.Razmi GR, Hossini M, Aslani MR. Identification of tick vectors of ovine theileriosis in an endemic region of Iran. Vet Parasitol. 2003;116:1–6. doi: 10.1016/s0304-4017(03)00254-1. [DOI] [PubMed] [Google Scholar]
- 5.Forsyth LMG, Jackson LA, Wilkie G, Sanderson A, Brown CGD, Preston PM. Bovine cells infected in vivo with Theileria annulata express CD11b, the C3bi complement receptor. Vet Res Commun. 1997;21:249–263. doi: 10.1023/a:1005886725717. [DOI] [PubMed] [Google Scholar]
- 6.Nalbantogiu ZS. Studies on the epidemiology of tropical theileriosis (Theileria annulata infection) in cattle in Central Anatolia Turkey. Trop Anim Health Produc. 2003;35:521–539. doi: 10.1023/a:1027348708038. [DOI] [PubMed] [Google Scholar]
- 7.Bakheit MA, Schnittger J, Salih DA, Boguslawski K, Beyer D, Fadl M, Ahmed JS. Application of the recombinant Theileria annulata surface protein in an indirect ELLSA for the diagnosis of tropical theileriosis. Parasitol Res. 2004;92:299–302. doi: 10.1007/s00436-003-1055-7. [DOI] [PubMed] [Google Scholar]
- 8.Radostits OM, Gay CC, Hinchcliff KW, Constable PD. Veterinary Medicine. 10th ed. Philadelphia, USA: W.B. Saunders Co; 2007. pp. 407–408.pp. 1526–1527. [Google Scholar]
- 9.Amadeo GP, Raplan LA. Methods in Clinical Chemistry. Vol. 2. Phildelphia, USA: W.B. Saunders Co; 1998. pp. 80–91. [Google Scholar]
- 10.Collins NE, Allsopp MT, Allsopp BA. Molecular diagnosis of theileriosis and heart water in bovines in Africa. Trans Roy Soc Trop Med Hyg. 2002;96(suppl 1):217–224. doi: 10.1016/s0035-9203(02)90079-9. [DOI] [PubMed] [Google Scholar]
- 11.Skoog DA, West DM, Holler FJ, Crouch SR. Analytic Chemistry: An Introduction. 7th ed. 2005. Flame photometric determination of sodium (Chapter 23) pp. 594–631. [Google Scholar]
- 12.Soulsby EJ. Helminth, Arthropoda and Protozoa of Domestic Animals. 7th ed. London, UK: Bailler Tindal Pub; 1986. pp. 734–740. [Google Scholar]
- 13.Tait A, Hall FR. Theileria annulata control measures diagnosis and the potential use of subunit vaccines. Rev Sci Tech. 1990:387–403. doi: 10.20506/rst.9.2.505. [DOI] [PubMed] [Google Scholar]
- 14.Forsyth LMG, Minns FC, Kirvar E, Adamson RE, Hall FR, McOrist S, Brown CGD, Preston PM. Tissue damage in cattle infected with Theileria annulata accompanied by metastasis of cytokine-producing, schizont-infected mononuclear phagocytes. J Comp Pathol. 1999;120:39–57. doi: 10.1053/jcpa.1998.0256. [DOI] [PubMed] [Google Scholar]
- 15.Glass EJ, Craigmile SC, Springbett A, Preston PM, Kirvar E, Wilkie GM, Eckersall PD, Hall FR, Brown CGD. The protozoan parasite, Theileria annulata, induces a distinct acute phase protein response in cattle that is associated with pathology. Int J Parasitol. 2003;33:1409–1418. doi: 10.1016/s0020-7519(03)00166-8. [DOI] [PubMed] [Google Scholar]
- 16.Graham SP, Brown DJ, Vatansever Z, Waddington D, Taylor LH, Nichani AK, Campbell JDM, Adamson RE, Glass EJ, Spooner RL. Proinflammatory cytokine expression by Theileria annulata infected cell lines correlates with the pathology they cause in vivo. Vaccine. 2001;19:2932–2944. doi: 10.1016/s0264-410x(00)00529-6. [DOI] [PubMed] [Google Scholar]
- 17.Beniwal RK, Sharma RD, Nichani AK. Determination of duration of immunity of calves vaccinated with the Theileria annulata schizont cell culture vaccine. Vet Parasitol. 2000;90:25–35. doi: 10.1016/s0304-4017(00)00226-0. [DOI] [PubMed] [Google Scholar]
- 18.Uilenberg G. Theileria infections other than east coast fever. Curr Top Vet Med Anim Sci. 1981;6:411–427. [Google Scholar]
- 19.Hilali M, Abdel-Gawad A, Nassar A, Abdel-Wahab A. Hematological and biochemical changes in water buffalo calves (Bubalus bubalis) infected with Trypanosoma evansi. Vet Parasitol. 2006;16:56–70. doi: 10.1016/j.vetpar.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Clark IA, Hunt NH, Cowden WB. Oxygen-derived free radicals in the pathogenesis of parasitic disease. Adv Parasitol. 1986;25:1–44. doi: 10.1016/s0065-308x(08)60341-3. [DOI] [PubMed] [Google Scholar]
- 21.Mbassa GK, Balemba O, Maselle RM, Mwaga NV. Severe anaemia due to haematopoietic precursor cell destruction in field cases of East Coast Fever in Tanzania. Vet Parasitol. 1994;52:243–256. doi: 10.1016/0304-4017(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 22.Yagi Y, Thongnoon P, Shiono H, Chikayama Y. Increase in oxidized proteins in Theileria sergenti-infected erythrocyte membrane. J Vet Med Sci. 2002;64:623–625. doi: 10.1292/jvms.64.623. [DOI] [PubMed] [Google Scholar]
- 23.Mehta HK, Sisodia RS, Misraulia KS. Clinical and haematological observations in experimentally induced cases of bovine tropical theileriosis. Indian J Anim Sci. 1998;58:584–587. [Google Scholar]
- 24.Sandhu GS, Grewal AS, Singh A, Kondal JK, Singh J, Brar RS. Haematological and biochemical studies on experimental Theileria annulata infection in crossbred calves. Vet Res Commun. 1998;22:347–354. doi: 10.1023/a:1006129306093. [DOI] [PubMed] [Google Scholar]
- 25.Singh A, Singh J, Grewal AS, Brar RS. Studies on some blood parameters of crossbred calves with experimental Theileria annulata infections. Vet Res Commun. 2001;25:289–300. doi: 10.1023/a:1010678625336. [DOI] [PubMed] [Google Scholar]
- 26.Rezakhani A, Papahn AA, Shekafrosh SH. Analysis of base apex lead electrocardiograms of normal dairy cows. Rev Vet Med. 2004;155:159. [Google Scholar]
- 27.Singh V, Amarpal P, Kinjavdekar HP, Aithal K. Medetomidine with ketamine and bupivacaine for epidural analgesia in buffaloes. Vet Res Commun. 2005;29:1–18. doi: 10.1023/b:verc.0000046736.78612.f7. [DOI] [PubMed] [Google Scholar]
- 28.Dudan F, Rossi GL, Luginbühl H. Cardiovascular study of the horse: relation between the vascular and tissue changes in the myocardium. Schweiz Arch Tierheilkd. 1985;127:369–378. [PubMed] [Google Scholar]