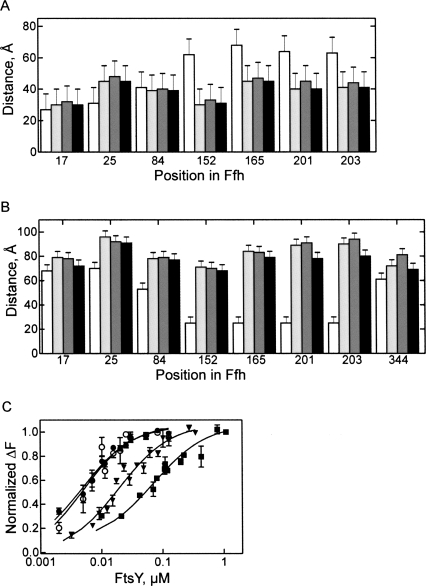
FIGURE 4.
FRET distances in SRP at different stages of targeting. FRET distances were measured in free SRP (white bars), SRP-70S (light gray), SRP-RNC (dark gray), or SRP-RNC-FtsY (black) complexes. (A) Distances between the indicated positions and position 344 in the M domain. (B) Distances from the indicated positions to the 3′ end of 4.5S RNA21–81. (C) Affinity of FtsY binding to SRP in different functional stages. Fluorescence titrations were carried out with SRP formed from Ffh(OG84) and full-length 4.5S RNA. Free SRP (▪); SRP bound to vacant 70S ribosomes (▼); SRP bound to Lep50-RNC (●); isolated Ffh(NG)(OG84) domain (○). Nonlinear fitting (continuous lines; see Materials and Methods) yielded the following K d values: FtsY-SRP, (70 ± 9) nM; FtsY-SRP-70S, (20 ± 3) nM; FtsY-SRP-RNC, (3 ± 0.1) nM; FtsY-Ffh(NG), (3 ± 0.2) nM.