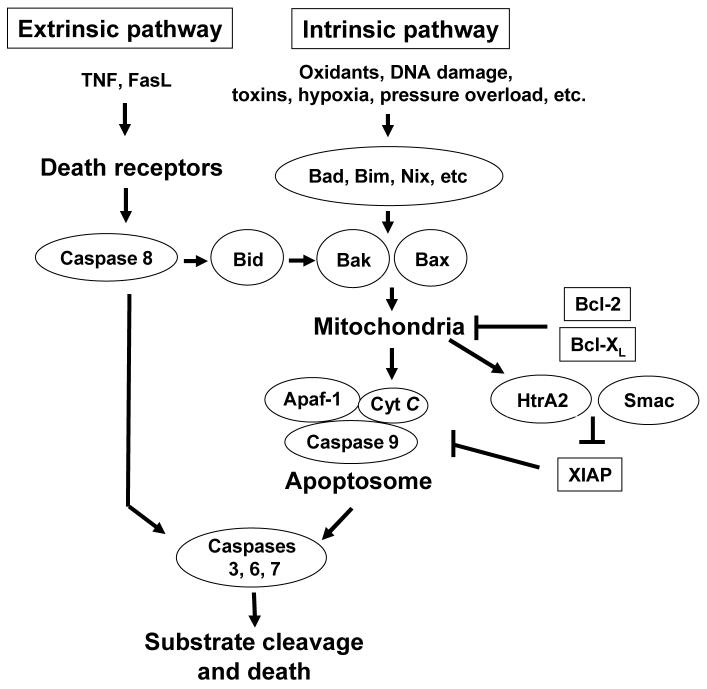
Figure 1. The extrinsic and intrinsic pathways of caspase activation.
In the extrinsic pathway, death ligands, such as FasL and TNFα, bind to their respective receptors, leading to caspase 8 activation. Activated caspase 8 directly activates procaspase 3, which then starts death cascade. Caspase 8 can also signal indirectly through the mitochondria via activation of Bid which translocates to mitochondria and activates Bax and Bak to trigger release of cytochrome c (Cyt C). In the intrinsic pathway, many stress stimuli activate caspases through the activation of BH3-only proteins of the Bcl-2 family, such as Bid, Bad, Bim, Nix, etc. The activated BH3-only proteins then activate Bax and Bak, leading to the release of cytochrome c as well as the release of Smac and HtrA2 from mitochondria. This process is antagonized by Bcl-2 and Bcl-XL. Cytochrome c forms a complex with the adaptor protein Apaf-1, and procaspase 9, resulting in the formation of apoptosome, which leads to the proteolytic cleavage and concomitant activation of caspase 9. Active caspase 9 directly cleaves and activates procaspase 3. This process is antagonized by XIAP, which can be inhibited by Smac and HtrA2 following their release from mitochondria.