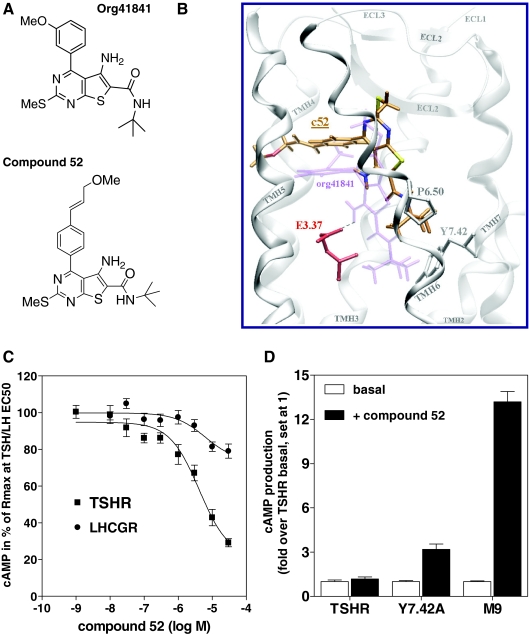
Figure 1.
TSHR antagonist NIDDK/CEB-52 (compound 52; c52): rational design and pharmacology. A, Structures of Org41841 and compound 52. B, Comparison of docking modes of compound 52 (C atoms orange) and Org41841 (light purple) in a model of TSHR. The binding pocket of compound 52 is within the extracellular half of the transmembrane helical bundle between TMH3, -5, -6, and -7 (white) and close to ECL2 (white). In contrast to Org41841, the t-butyl group of compound 52 sits higher in the binding cleft and points toward P6.50. Therefore, movement of TMH6 during TSHR activation is not enforced by compound 52. This structural constraint may cause an antagonistic effect on TSHR signaling (C). Noteworthy, experimental data reveal that in the TSHR single mutant Y7.42A, compound 52 acts as an agonist (D). In Y7.42A, the alanine is less bulky compared with tyrosine; therefore, compound 52 moves downward to TMH6 and TMH7, similar to Org41841, and presses the kinked TMH6 apart below P6.50, which likely leads to movement of the intracellular part of TMH6 and TSHR activation. C, Antagonistic activity of compound 52 at TSHR and LHCGR. Intracellular cAMP accumulation was determined in response to increasing concentrations of compound 52. EC50 concentrations of native ligands were as follows: TSH, 1.8 nm; LH, 0.34 nm. D, Compound 52 activates TSHR mutants Y7.42A and M9 in contrast to TSHR. Intracellular cAMP accumulation was determined without ligands (basal) or in response to 30 μm of compound 52. In M9 (7); TSHR residues within and covering the Org41841 binding cleft were replaced by the corresponding residues of LHCGR: I560V (LHCGR: V505), L570F (LHCGR: F515), P5.34T, A5.36S, L5.37Q, A5.38V, F5.42T, Y6.54F, and I5.59A. The data are presented as mean ± sem of three independent experiments, each performed in duplicate.