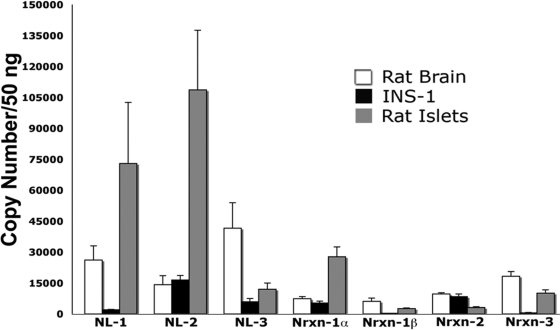
Figure 1.
Abundance of neuroligin and neurexin transcripts in INS-1 β-cells and rat islets. Absolute real-time qPCR analysis was used to compare the expression levels of individual neuroligin (NL) and neurexin (Nrxn) family members within rat-derived INS-1 β-cells, rat islets, and rat brain. Standard curves were generated using plasmids encoding either whole or partial neuroligin/neurexin sequences, and these were used to calculate the number of copies of each transcript per cDNA sample. Samples were prepared in parallel, though rat islet RNA was prepared separately because of the need to use an alternative method. Each cDNA sample represents an equal amount (50 ng) of input total RNA. In INS-1 cells, neuroligin-2 (16,489 ± 1,055 copies/50 ng RNA), neurexin-1α (5,296 ± 927 copies/50 ng RNA), and neurexin-2 (8,370 ± 1,302 copies/50 ng RNA) are expressed at the highest levels relative to other neuroligin and neurexin family members. (The neurexin-2 and -3 primer sets could not distinguish between the α and β-forms.) In rat islets, neuroligin-1 (72,914 ± 14,836 copies), neuroligin-2 (108,683 ± 14,413 copies), neurexin-1α (27,668 ± 2,417 copies), and neurexin-3 (10,115 ± 769 copies) are expressed at the highest levels relative to other neuroligin and neurexin family members. In rat brain, expression of neuroligins and neurexin mRNA is more evenly distributed. Values obtained were: neuroligin-1 (15,058 ± 3,987 copies); neuroligin-2 (9,260 ± 2888 copies); neuroligin-3 (26,156 ± 8,018 copies); neurexin-1α (4,412 ± 1,077 copies); neurexin-1β (3,464 ± 883 copies); neurexin-2 (6,451 ± 1,552 copies); and neurexin-3 (11,239 ± 2,727 copies). Equal loading of input RNA was verified by parallel qPCR analysis of 18s rRNA. 18s cycle threshold values (CT) in INS-1 cells and rat brain were consistently identical. Rat islet 18s cycle threshold values were higher (slightly less than two CT values), likely the result of differences in the technique used for RNA isolation. (n of 3 separately prepared RNA/cDNA samples per experiment).