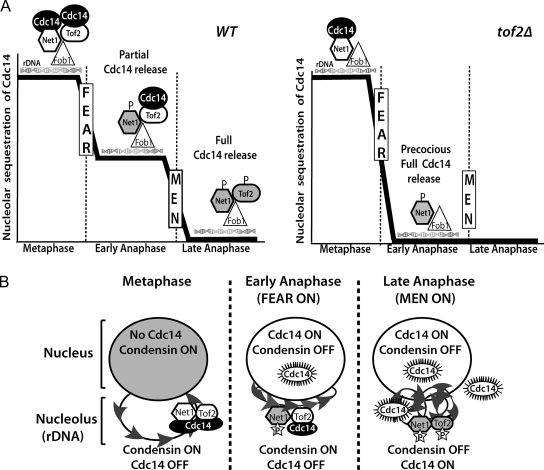
Figure 6.
Models for the biphasic release of Cdc14 and its role in rDNA condensation. (A) In WT cells (left), Cdc14 is restricted to the ribosomal DNA through interactions with its inhibitor, Net1, as well as the Cdc14-binding protein Tof2. Both proteins coimmunoprecipitate with Fob1, the RFB-binding protein. In response to FEAR network activation in early anaphase, Net1 phosphorylation promotes a partial release of Cdc14 into the nucleus. Cdc14 remaining in the nucleolus is dependent on Tof2, which may function by either directly restraining a population of Cdc14 within the nucleolus or alternatively, could inhibit Cdc14 release from Net1 in a subpopulation of RENT complexes. In late anaphase, the MEN promotes the full release of Cdc14, possibly through phosphorylation of Tof2. In the absence of Tof2 (right), FEAR network activation promotes the full release of Cdc14, independently of the MEN. Note that for simplicity, only a subset of Net1- and Tof2-interacting factors are included in the schematic. (B) Model for the spatiotemporal regulation of condensation during mitosis. In metaphase, Cdc14 activity is inhibited throughout the nucleus and nucleolus by sequestration and binding to its inhibitor Net1. In early anaphase, Net1 phosphorylation in the nucleolus causes the partial release of active Cdc14 into the nucleus, which promotes condensin enrichment to the nucleolus and antagonizes its activity in the nucleus. Tof2 bound to the rDNA serves as a second Cdc14 sequestration activity, thereby promoting condensin dependent hypercondensation of the rDNA. In late anaphase, full release of Cdc14 throughout the nucleolus and the cell favors rDNA decondensation and a resetting of the cell cycle to the G1 state.