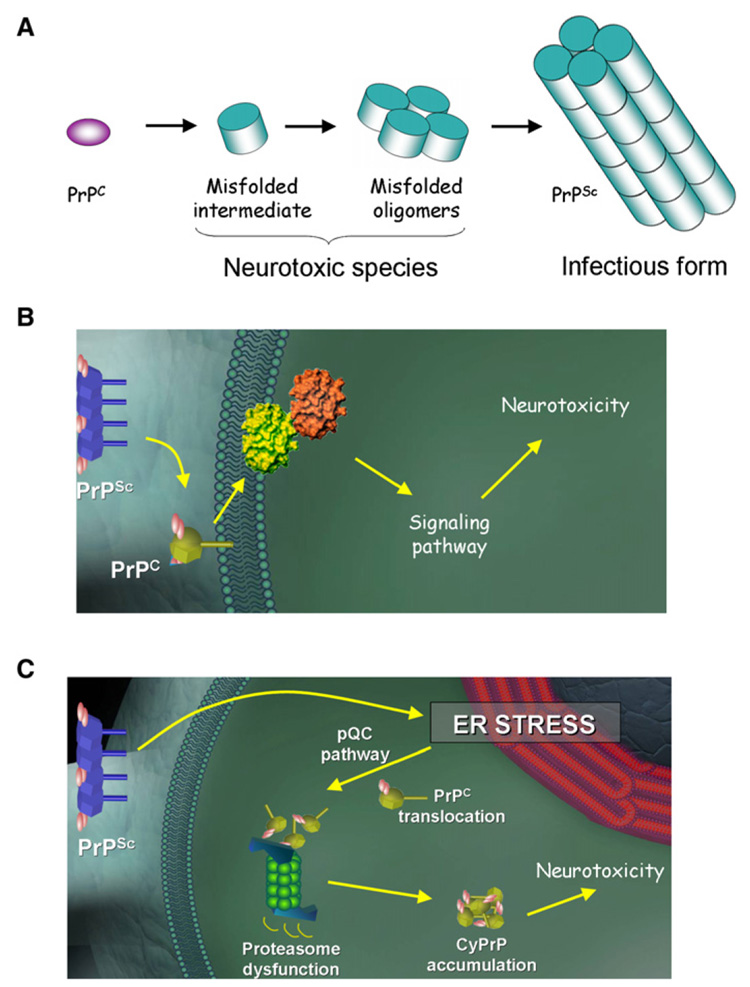
Figure 1. Role of PrPSc and Mechanism of Neurodegeneration.
Although PrPSc is certainly associated to the pathogenesis of TSEs, its role is neurodegeneration is controversial. Several observations suggest that PrPSc levels do not correlate with the extent of brain damage and that the natively folded PrPC is required for neurodegeneration. Three non-mutually exclusive models can be proposed to explain the discrepancies and the implication of PrPC.
(A) First, the infectious and neurotoxic PrP species might not be the same. Indeed, it is possible that an undetectable misfolded intermediate might be responsible for neurotoxicity in a way similar to that proposed for other neurodegenerative diseases. The transient nature of these intermediates determines that its presence depends on the permanent synthesis of new PrPC.
(B) PrPC located in the cell surface may act as a receptor for PrPSc, triggering a signal transduction pathway leading to neurodegeneration.
(C) As proposed by Rane and colleagues in this issue (Rane et al., 2008), induction of ER stress by PrPSc may lead to translocation of nascent PrPC molecules to the cytosol for proteasomal degradation as a way to alleviate the damaged ER (pQC pathway). However, this mechanism of defense turns negative under chronic ER stress conditions, overwhelming the proteasome and leading to the cytosolic accumulation of potentially toxic PrP molecules.