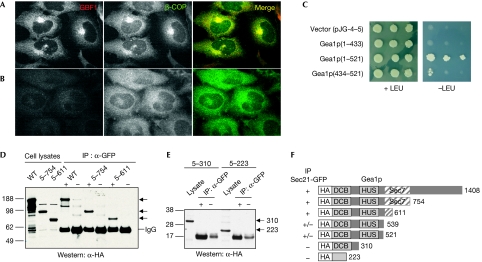
Figure 1.
Functional and physical interaction between GBF/Gea Arf guanine nucleotide exchange factors and the COPI coat. (A,B) Specificity of recruitment of COPI by GBF1 in vivo. HeLa cells were transfected with short interfering RNAs targeting (A) BIG1+BIG2, or (B) GBF1 and prepared for immunofluorescence analysis using antibodies against GBF1 and β-COP. (C) Yeast two-hybrid interaction between the yeast GBF1 homologue, Gea1p, and yeast γ-COP, Sec21p. Bait plasmid carrying full-length Sec21p was co-transformed into yeast with prey plasmid alone or carrying the indicated regions of Gea1p. Cells were grown on control (+LEU) and selective medium (−LEU) to monitor the expression of the reporter. (D) Lysates from yeast strain CJY104 SEC21∷3xGFP carrying a low-copy plasmid expressing HA-Gea1p, HA-Gea1p(5–754) or HA-Gea1p(5–611), were prepared and incubated with GFP antibodies (+) or IgG alone (−). Bound material was analysed by Western blot using HA antibodies. (E) Same as (D) except CJY104 carried plasmids expressing HA-Gea1p(5–310) or HA-Gea1p(5–223). (F) Summary of co-IPs using indicated truncations of Gea1p. Arf, ADP-ribosylation factor; BIG, brefeldin A-inhibited guanine nucleotide exchange protein; co-IP, co-immunoprecipitation; COPI, coat protein complex I; DCB, dimerization and cycophilin-binding domain; GBF1, Golgi-associated brefeldin A-resistant guanine nucleotide exchange factor 1; Gea, guanine nucleotide exchange on Arf; GFP, green fluorescent protein; HA, haemagglutinin; HUS, homology upstream from Sec7 domain; Sec7, catalytic domain; WT, wild type.