Abstract
OBJECTIVE: To investigate tolerability and glycemic control over 26 weeks in patients with type 2 diabetes (T2D) who initiated insulin with, or switched to, biphasic insulin aspart 30/70 (BIAsp 30) in routine clinical care. METHODS: This was a non-randomized, non-interventional, open-label, observational study involving patients under the care of approximately 150 insulin-prescribing physicians in Denmark. All patients enrolled were prescribed BIAsp 30 in routine care. Starting dose, dose titration and injection frequency were determined individually by each physician. Information on serious adverse drug reactions (SADR), glycemic parameters and hypoglycemic events were obtained from patients’ notes, patients’ diaries and recall, and transferred to case report forms by physicians at baseline (during 4 weeks prior to BIAsp 30 therapy) and after 12 and 26 weeks of treatment. RESULTS: 421 subjects were recruited and 392 provided safety data. The age (mean ± SD) was 62.0 ± 11.4 years, body mass index (BMI) 30.4 ± 6.4 kg/m2, duration of diabetes 9.1 ± 8.1 years and HbA1c (%) 9.4 ± 1.7. 199 subjects were prior insulin users and 193 were insulin-naïve patients. Four patients reported a SADR (3 hypoglycemia, 1 severe hypoglycemia). HbA1c was significantly reduced after 26 weeks of BIAsp 30 therapy: prior insulin users -1.2%, insulin-naïve patients -2.2% (both p < 0.001). 28% and 41% of patients, respectively, reached target HbA1c < 7%. Overall the hypoglycemia rate was lower for insulin-naïve patients than for prior insulin users: 5.0 vs. 6.6 episodes/patient-year (p < 0.05). CONCLUSION: Initiating insulin with, or switching insulin to, BIAsp 30 in routine care was safe and effective in patients with T2D.
Keywords: type 2 diabetes, human insulin, glycemic control, biphasic insulin aspart, BIAsp, HbA1c
Introduction
The achievement of glycemic targets and minimizing the risks of diabetes-related complications are major goals of diabetes management [1]. However, once targets have been reached, maintaining these levels of control in the face of progressing disease is a challenge for patients with type 2 diabetes and their physicians.
After diet and lifestyle changes, many patients with type 2 diabetes are prescribed oral antidiabetic drugs (OAD). When taken as monotherapy, their benefit is relatively short-lived. After 3 years, approximately 50% of patients are unable to maintain glycosylated hemoglobin (HbA1c) below 7% [2], the American Diabetes Association recommended HbA1c target [3]. At this juncture, another class of OAD can be added to the treatment regimen, or insulin therapy can be initiated.
Because of the well-documented glucose-lowering power of exogenous insulin, many patients with type 2 diabetes progress to insulin therapy after the failure of OAD. Basal insulin is often the first type of insulin prescribed, with studies showing good results for neutral protamine Hagedorn (NPH) insulin and basal insulin analogs in patients previously insulin-naïve but still taking OAD [4-7].
However, clinical trials have shown that initiating insulin with the modern premixed insulin, biphasic insulin aspart 30/70 (BIAsp 30: 30% insulin aspart, 70% protaminated aspart) twice-daily (BID) can be more efficacious than initiating with the basal analog, insulin glargine [8, 9]. In the INITIATE study, HbA1c reduction after 28 weeks was significantly greater with BIAsp 30 BID therapy than with insulin glargine once daily (OD), -2.79% vs. -2.36%, respectively (p < 0.01). Furthermore, HbA1c reductions were even larger in patients with poor HbA1c at baseline (i.e., >8.5%). This was due to 25% lower postprandial glycemic exposure with BIAsp 30, since fasting plasma glucose (FPG) reductions were similar for both insulins [8]. In these studies, major hypoglycemia and serious adverse events were rarely reported with both BIAsp 30 and insulin glargine [8, 9]. Such tolerability, together with the potential to improve glycemic control, makes BIAsp 30 an attractive option for patients currently treated with conventional biphasic human insulins.
Several pharmacological studies have shown that BIAsp 30 is absorbed faster and produces a higher peak plasma insulin level than does biphasic human insulin 30/70 (BHI 30) [10-12]. Consequently, BIAsp 30 provides greater postprandial glycemic control, with the added benefit of lower reported rates of major hypoglycemia, compared with human premix in patients with type 2 diabetes already using insulin [13-15].
It is important to know if these results with BIAsp 30 from randomized controlled trials (RCT) are achievable in daily routine practice in hospital outpatient clinics and primary care clinics. For this purpose, observational studies are a valuable tool for collecting real-life data to complement those from RCT. The aims of this observational study were to investigate the frequency of serious adverse drug reactions (SADR), including major hypoglycemia, and glycemic control over 26 weeks in patients with type 2 diabetes initiating insulin with, or switching to, BIAsp 30 as part of routine clinical care.
Methods and materials
Patients and study design
This was an open-label, observational study involving patients with type 2 diabetes in the care of 141 insulin-prescribing physicians in Denmark, either in a hospital or a primary care setting. The study was conducted in accordance with the Declaration of Helsinki.
Insulin-naïve patients who started insulin therapy with BIAsp 30 and patients who switched from other insulin regimens to BIAsp 30 by prescription of their physician as part of routine clinical practice in order to improve glycemic control were eligible for the study. The selection of patients was at the discretion of the treating physician.
Treatment of patients with BIAsp 30
BIAsp 30 was prescribed by the physicians as part of normal clinical evaluation. The starting dose and injection frequency were determined individually by each physician, as well as later changes to either dose or injection frequency.
Physicians evaluated their patients at the following three routinely scheduled clinic visits:
Baseline visit (before initiating BIAsp 30 treatment).
Interim visit (approximately 12 weeks after initiating BIAsp 30 treatment).
Final visit (approximately 26 weeks after initiating BIAsp 30 treatment).
Data collection
Information from patient recall, patients’ notes and patients’ self-monitored blood glucose diaries were collected on case report forms (CRF) by physicians at baseline (during 4 weeks prior to starting BIAsp 30 therapy) and at week 12 and week 26 of BIAsp 30 treatment.
Endpoints
The primary endpoint was the incidence of SADR leading to death, a life-threatening experience, hospitalization or significant disability/incapacity, including major hypoglycemia, during BIAsp 30 therapy. Episodes of major hypoglycemia were defined as events with severe central nervous system symptoms in subjects unable to treat themselves, accompanied by plasma glucose < 3.1 mmol/l or reversal of symptoms after food, glucagon injection or intravenous glucose. This analysis was carried out using the safety population (i.e. all patients who received at least one dose of BIAsp 30 and returned safety data).
Secondary endpoints were changes from baseline in HbA1c and mean FPG at 12 and 26 weeks, and overall, daytime and nocturnal frequency of hypoglycemic events (major and minor), summarized at baseline, 12 weeks and 26 weeks. A minor hypoglycemic event was defined as having one of the following characteristics: symptoms of hypoglycemia that resolved with oral carbohydrate or any plasma glucose measurement <3.1 mmol/l, regardless of symptoms. Nocturnal hypoglycemic events were defined as symptomatic events occurring during sleep, between bed-time and rising in the morning.
Hypoglycemia data were collected over 4 week periods prior to each visit. SADR and hypoglycemia analyses were carried out using the safety population, equal to the intention-to-treat (ITT) population. Efficacy analyses were carried out using the on-treatment population.
Statistical analyses
Participants were stratified according to prior insulin use (prior insulin users and insulin-naïve). The upper 95% confidence limits were estimated for the primary endpoint, but no significance testing was performed. Statistical analyses were performed on changes from baseline of HbA1c and FPG at each visit using the paired t-test. Statistical analyses on rates of hypoglycemic events were performed between subgroups, using a test of proportions.
Results
Patient flow
Of the 421 participants recruited, 29 failed to provide safety data, leaving 392 participants in the safety (also the ITT) population (199 insulin-treated; 193 previously insulin-naïve). A further 11 participants were eliminated for failing to provide efficacy data, leaving 381 participants in the on-treatment population. A total of 352 participants completed the study. A diagram of patient flow is shown in Figure 1.
Figure 1. Diagram of patient flow through the study.
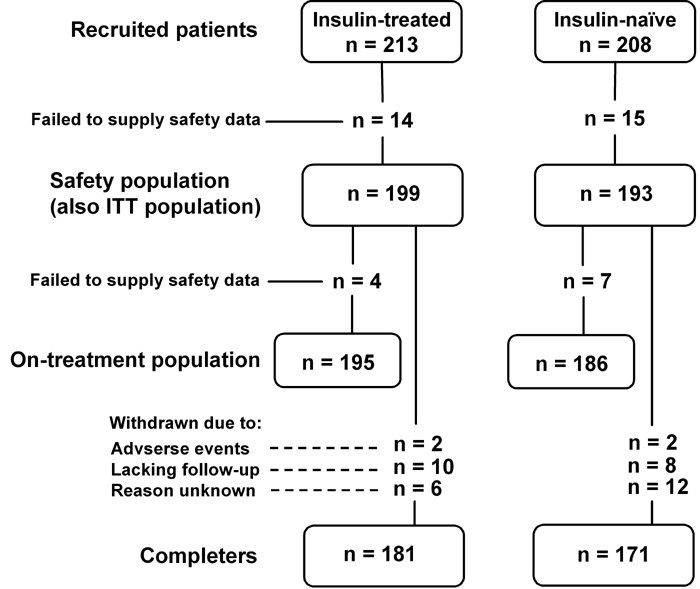
ITT: intention-to-treat, n: number of patients.
Demographics and baseline characteristics
Demographics and baseline characteristics were similar between prior insulin users and insulin-naïve patients, with the exception that diabetes duration was almost twice as long in prior insulin users than observed in insulin-naïve patients, as would be expected (Table 1). Overall, 30.4% of patients came from primary care.
Table 1. Demographics and baseline characteristics of prior insulin users and insulin-naïve patients.
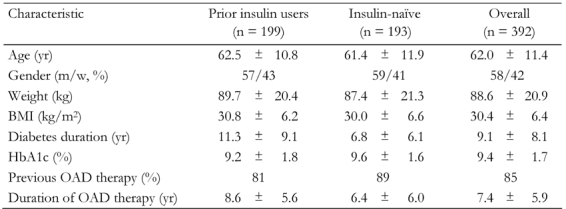
Data are mean ± SD. m: men. w: women. BMI: body mass index. OAD: oral antidiabetic drug.
At the time of starting BIAsp 30 therapy, 234 patients (prior insulin users and insulin-naïve patients combined) were also taking OAD. The most frequently used were biguanides (85% of patients), sulfonylureas (22%) and thiazolidinediones (1%). This prescribing pattern was similar at the final visit. For patients previously using insulin, the vast majority (97%) were taking just one OAD at the start of the study. For insulin-naïve patients, 87% were taking one OAD and 13% two OAD at the start of the study.
The most common insulins used prior to the start of BIAsp 30 therapy were NPH insulin (63% of patients) and human premixed insulin (22%). The remaining participants were on insulin analogs. The mean (SEM) total daily insulin dose was 40.7 (28.4) IU. At the initiation of BIAsp 30 therapy (baseline visit), three out of four patients (74%) were not given a glycemic target by their physician. The most common reason for physicians to start patients on BIAsp 30 therapy was to improve glycemic control (88% of patients).
Incidence of serious adverse drug reactions (SADR)
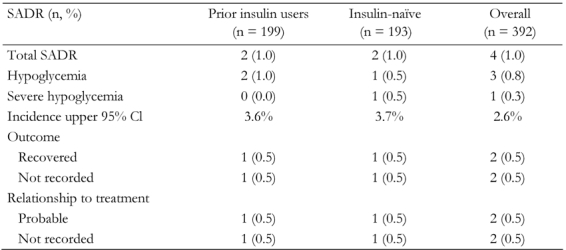
Four patients reported a SADR, two (1% of the cohort) in the prior insulin users group and two (1%) in the insulin-naïve group. Of these, three reported their events to be 'hypoglycemia' and the fourth (insulin-naïve) reported ‘severe hypoglycemia’ (Table 2).
Table 2. Incidence of serious adverse drug reactions (SADR) in prior insulin users and insulin-naïve patients.
HbA1c levels
HbA1c was significantly reduced from baseline after both 12 and 26 weeks of BIAsp 30 therapy, for both prior insulin users and insulin-naïve patients (Figure 2). The mean (SEM) HbA1c reduction at the end of the study was -1.2 (0.13)% for prior insulin users and -2.2 (0.15)% for the insulin-naïve group.
Figure 2.
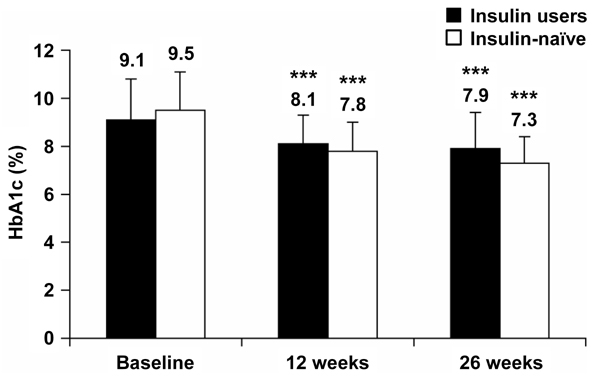
HbA1c levels at baseline and after 12 and 26 weeks of BIAsp 30 therapy, for prior insulin users and for previously insulin-naïve patients. Error bars are SD. ***p < 0.001 compared with baseline.
Both insulin-naïve and prior insulin users with HbA1c ≥ 8.5% at baseline reported larger HbA1c reductions from baseline at 12 and 26 weeks than the overall group cohorts. For insulin users, with HbA1c ≥ 8.5%, HbA1c reductions (mean ± SEM) were -1.4 ± 0.16 (p < 0.001) at 12 weeks and -1.7 ± 0.17 (p < 0.001) at 26 weeks. For insulin-naïve patients, with HbA1c ≥ 8.5% at baseline, HbA1c reductions (mean ± SEM) were -2.3 ± 0.16 (p < 0.001) at 12 weeks and -2.9 ± 0.15 (p < 0.001) at 26 weeks. The difference between insulin users and insulin-naïve patients was statistically significant at 12 and 26 weeks for those with baseline HbA1c ≥ 8.5% only. The mean difference at 12 weeks was 0.83%, (95% CI: 0.38-1.28%) and at 26 weeks was 1.17% (95% CI: 0.73-1.61%).
HbA1c reductions in patients with baseline HbA1c < 8.5% were smaller than in those with baseline HbA1c ≥ 8.5% at both 12 and 26 weeks. Specifically, HbA1c reduction of insulin users, with HbA1c < 8.5%, was -0.23 ± 0.10 (p < 0.05) at 12 weeks and -0.34% (0.10) (p < 0.01) at 26 weeks. Insulin-naive patients, with HbA1c < 8.5%, experienced a HbA1c reduction of -0.35 ± 0.13 (p < 0.01) at 12 weeks and -0.44 ± 0.14 (p < 0.01) at 26 weeks. The large group of NPH insulin-treated patients who switched to BIAsp 30 had a significant improvement in HbA1c at 26 weeks when compared with baseline levels (-1.23%, p < 0.001). HbA1c (mean ± SD) at baseline was 9.2 ± 1.7 and after 26 weeks 7.9 ± 1.3.
Overall, the proportion of patients reaching the HbA1c target of < 7.0% at 26 weeks was significantly higher for insulin-naïve patients: 28% of insulin users vs. 41% of insulin-naïve patients (difference: 13%, 95% CI: 3-24%) (Figure 3).
Figure 3.
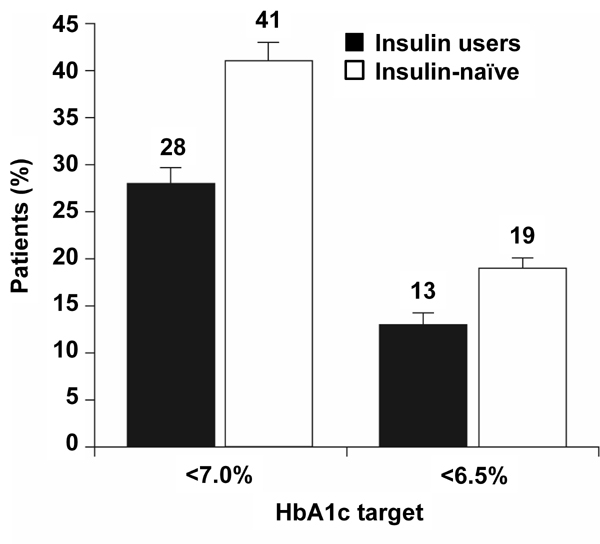
The proportions of prior insulin users and insulin-naïve patients who reached HbA1c targets <7.0% and <6.5% after 26 weeks of BIAsp 30 therapy. Error bars are SE.
Patients attending hospital outpatient clinics had higher HbA1c at baseline than patients in primary care settings (Table 3), but prior insulin users and insulin-naïve patients in both groups showed significantly improved HbA1c at the final visit. As in the overall population, HbA1c reductions were greater in insulin-naïve patients than in prior insulin users in both settings (Table 3).
Table 3. HbA1c changes after BIAsp 30 therapy for prior insulin users and insulin-naïve patients in hospital outpatient clinics and primary care settings.
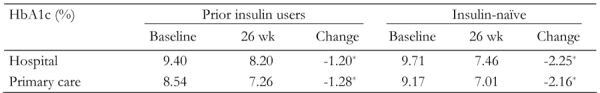
* p < 0.05.
Fasting plasma glucose
Mean self-measured FPG levels decreased from baseline after both 12 weeks and 26 weeks of BIAsp 30 therapy, for prior insulin users and insulin-naïve patients (Figure 4). Changes in FPG (mean ± SEM) from baseline were significantly larger for insulin-naïve patients. FPG levels for prior insulin users were -0.3 ± 0.4 mmol/l at 12 weeks (not statistically significant), and -1.1 ± 0.4 mmol/l at 26 weeks (p < 0.01). FPG levels for insulin-naïve patients were -3.3 ± 0.4 mmol/l (p < 0.001) at 12 weeks and -3.7 ± 0.4 mmol/l (p < 0.001) at 26 weeks.
Figure 4.
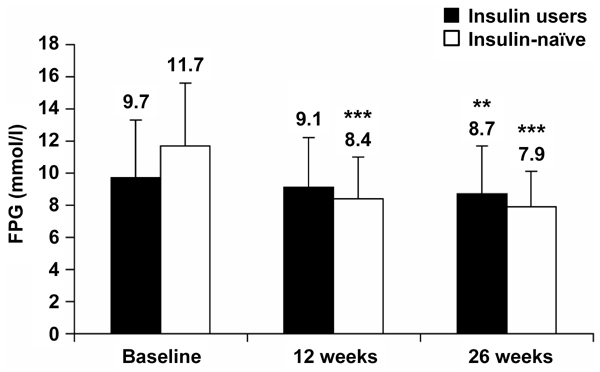
Self-measured fasting plasma glucose (FPG) levels at baseline and after 12 and 26 weeks of BIAsp 30 therapy, for prior insulin users and for previously insulin-naïve patients. Error bars are SD. **p < 0.01, ***p < 0.001.
Hypoglycemia
The proportion of patients reporting any hypoglycemia (any time of day) was lower at baseline for insulin-naïve patients than for prior insulin users: 5% vs. 13% of patients, respectively. At the end of the study, the proportion was similar between groups: 14% and 17%, respectively.
Hypoglycemia incidence and rate were reportedly lower at night than during the daytime in both groups (Table 4). However, hypoglycemia rates were also significantly lower at night-time (and hence in total) for insulin-naïve patients compared with the prior insulin users (Table 4). Major hypoglycemia was extremely rare in both groups. There were no episodes of major hypoglycemia at baseline in either group, and only 1% of prior insulin users and insulin-naïve patients reported major episodes at 12 weeks and 26 weeks of therapy with BIAsp 30. Neither group reported any major hypoglycemia at night-time.
Table 4. Incidence and rate of all hypoglycemia for prior insulin users and previously insulin-naïve patients, during the daytime and night-time (measured during 4 weeks prior to the final visit).
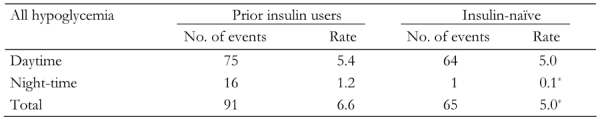
* p < 0.05.
Weight change
Over the 26 week observation period, both prior insulin users and insulin-naïve patients gained weight (Figure 5). Changes from baseline at 26 weeks were +1.0 kg (p < 0.01) and +1.4 kg (p < 0.01) for prior insulin users and insulin-naïve patients, respectively. Regression analyses for the relationship between body weight change from baseline and baseline BMI demonstrated a negative correlation: those with the lowest BMI gained relatively more weight than those with a high baseline BMI (p < 0.001) Also, no relationship was found between baseline BMI or prior insulin use and HbA1c outcome.
Figure 5.
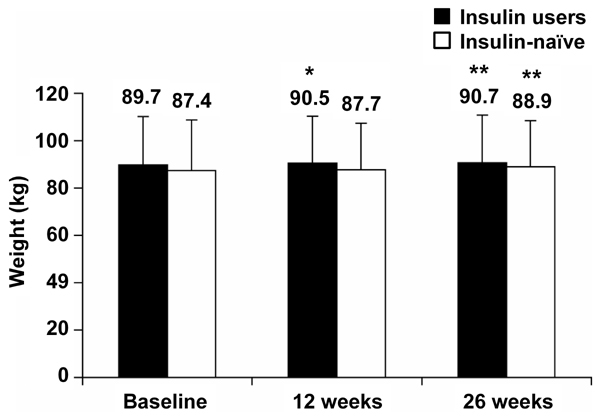
Patient weight at baseline and after 12 and 26 weeks of BIAsp 30 treatment, for prior insulin users and previously insulin-naïve patients. p-values refer to differences from baseline. Error bars are SD. *p < 0.05, **p < 0.01.
Insulin dose
The total daily dose of BIAsp 30 increased from baseline at 12 and 26 weeks, in both prior insulin users and insulin-naïve groups. Insulin dose at all three visits was significantly higher in prior insulin users than in previously insulin-naïve patients (Table 5).
Table 5. Total daily biphasic insulin aspart 30 (BIAsp 30) dose at baseline and after 12 and 26 weeks’ therapy in patients previously using insulin and patients previously insulin-naïve.
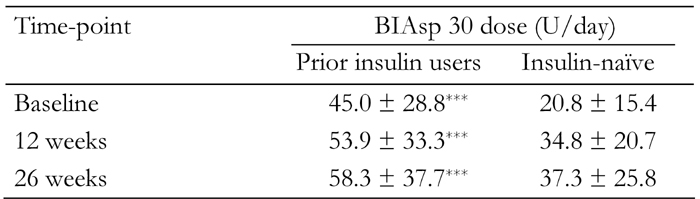
Data are mean ± SD. *** p < 0.001.
Among the insulin users, 66% injected BIAsp 30 twice-daily (BID) at baseline, with 24% injecting once daily (OD) and 10% three-times-daily (TID). At the final visit, the proportion of insulin users injecting BIAsp 30 BID remained unchanged (66%), while 12% injected OD and 22% TID.
Among the insulin-naïve patients, the most common injection frequency at baseline was OD (58%), with 39% injecting BID and 3% TID. At the final visit, 67% of this group were injecting BIAsp 30 BID, with 25% injecting OD and 8% TID.
Regression analysis showed that insulin dose was positively correlated with HbA1c over time, such that increased HbA1c was associated with increased dose (0.008%/unit insulin; p < 0.001). Thus, while mean HbA1c decreased from baseline at 12 and 26 weeks, patients with a higher HbA1c at each time point received larger insulin doses than those with a lower HbA1c.
At the final visit, greater insulin dose was also significantly correlated with higher body weight for prior insulin users and insulin-naïve patients: both 0.21 kg/unit insulin; p < 0.001 and p < 0.01, respectively.
Discussion
While RCT are perceived as the 'gold standard' in clinical investigation, observational studies such as this have certain features making them valuable tools for providing complementary data to those from RCT. Some of the features include fewer restrictions on patient numbers, the opportunity to study numerous outcomes, and the applicability of results to a broader patient population [16]. The observational study therefore offers an insight into clinical practice and outcomes.
In the present observational study, initiating insulin therapy or switching existing insulin therapy to BIAsp 30 proved safe and effective, with only 1% of patients reporting an SADR. This compares favorably with data from RCT. In a 6 month comparison of twice-daily BIAsp 30 plus metformin vs. once-daily insulin glargine plus glimepiride, 7.8% of insulin-naïve patients with type 2 diabetes in the BIAsp group reported a SADR, compared with 8.7% in the glargine group [9]. Consistent with our findings, a large, international observational study, PRESENT, also found very low rates of adverse events associated with BIAsp 30 therapy. Adverse drug reactions occurred at a rate of 0.05 events per patient-year and only 13 SADR were reported over a 6 month period in a population of almost 22,000 patients [17].
In our study, with patients taking BIAsp 30 therapy, glycemic control (measured by HbA1c and FPG) improved significantly for both prior insulin users and previously insulin-naïve patients during the observational period. The majority of improvement in both HbA1c and FPG occurred during the first 12 weeks of BIAsp 30 therapy, with further improvement up to 26 weeks. Predictably, patients who were previously insulin-naïve showed larger reductions in HbA1c and FPG than those who were previously insulin users, resulting in significantly more insulin-naïve patients reaching target HbA1c < 7.0% (41% vs. 28%, respectively).
Similar results have been found in an RCT of insulin-naïve patients with type 2 diabetes (the 4T study [5]) and in the larger PRESENT observational study [17]. Of the 235 patients in the 4T study randomized to receive BIAsp 30 BID (where a further 239 patients were randomized to prandial insulin analog and 234 to basal insulin analog), 41.7% achieved the target HbA1c < 7.0% with regular dose titration to target glycemic levels. In the PRESENT study, patients who were treatment-naïve or insulin-naïve (oral therapy only) achieved 6 month HbA1c reductions of -2.35% and -2.15%, respectively, while those who were previously using insulin alone, or with oral therapy, achieved reductions of -1.45% and -1.47%, respectively [17].
Rates of overall hypoglycemia in our observational study were very similar to that found in the 4T study described earlier [5] (using similar criteria to define hypoglycemia), confirming that results from RCT transfer to routine clinical practice. Our rates of hypoglycemia were 5.0 and 6.6 events per person-year (for insulin-naïve and prior insulin users, respectively), compared with 5.7 events per person per year for BID dosing of BIAsp 30 in the 4T study [5].
Also consistent with data from clinical trials, the occurrence of major hypoglycemia with BIAsp 30 was very low in this observational study, and no events were reported to occur at night. In a two-year RCT of BIAsp 30 vs. BHI 30, patients with type 2 diabetes reported a total of only 3 events of major hypoglycemia during the first year, and no events during the second year [14]. Similarly, a 32 week crossover study of these same premix insulins reported only 2 episodes of major hypoglycemia associated with BIAsp 30 therapy, among a study population of 160 people with type 2 diabetes [18].
In the present study, the rate of overall hypoglycemia was higher during the day than at night for both groups, supporting the findings of the above-mentioned study [18] which, as well as patient self-reporting, used continuous glucose monitoring to record episodes of low interstitial glucose (IG) in BIAsp 30-treated patients with type 2 diabetes. Here, the daytime rate with BIAsp 30 therapy was 2.6 episodes (IG < 3.5 mmol/l) per patient-week compared with 1.2 episodes at night.
In our study, hypoglycemia was also significantly lower at night in insulin-naïve patients than in prior insulin users (0.1 vs. 1.2 events/patient-year). This is contrary to the findings in the PRESENT observational study, in which end-of-study hypoglycemia rates were less than 1 event/patient year in all pre-study treatment groups. Our findings may therefore represent differences in the study populations and/or the large difference in daily BIAsp 30 dose between prior insulin users and insulin-naïve patients. The mean BIAsp 30 dose at 26 weeks for prior insulin users was almost 50% larger than that for the insulin-naïve group, possibly reflecting greater residual beta-cell function in the insulin-naïve patients, which in turn has been suggested to reduce the risk of severe hypoglycemia [19]. It must be stressed that the higher rate of 1.2 events/patient-year observed in prior insulin users is still very low.
Despite the difference in dosing, baseline and end-of-study weights were similar for insulin-naïve patients and prior insulin users. Weight gain was modest in both groups: 1.4 and 1.0 kg, respectively. This is consistent with the PRESENT observational study involving 17,946 patients who returned data after 6 months’ BIAsp 30 therapy. Regardless of pre-study therapy (insulin/naïve), the mean final visit weight for all patient groups was within 1 kg of the baseline weight [17].
Weight gain associated with BIAsp 30 therapy is often higher in clinical trials than in observational studies. This may be because the insulin dose is titrated towards a treatment target. While this may produce impressive glycemic control, the resulting high insulin dose can lead to increased weight gain. In the 1-2-3 study [20], patients were started on BIAsp 30 with a once-daily injection for 16 weeks, increasing to two and then three injections if the glycemic target (HbA1c < 6.5%) was not met. The mean daily dose increased from 0.6 U/kg for once-daily BIAsp 30 to 1.52 U/kg, with a concomitant increase in weight of 5 kg [20]. A similar amount of weight gain was observed during the ACTION study, when BIAsp 30 was added to an optimized OAD regimen in patients with type 2 diabetes [21]. Again, an insulin dose titration algorithm was used, resulting in 76% of BIAsp 30-treated patients achieving HbA1c < 7.0%, however their body weight increased by an average of 4.6 kg [21]. Dose adjustment in our observational study was done at the discretion of the individual physicians. The results, which reflect routine clinical practice in Denmark, demonstrate that weight gain need not be a barrier to initiating insulin therapy in patients with type 2 diabetes.
Conclusions
The benefits of BIAsp 30 therapy demonstrated in randomized, clinical trials are apparent in a real clinical setting. Initiating insulin with, or switching insulin therapy to, BIAsp 30 is safe and effective in patients with type 2 diabetes. Improvement in glycemic control was substantial in previously insulin-naïve patients, as well as in prior insulin users. Since the majority of prior insulin users were injecting human basal insulin, these data suggest that intensification with BIAsp 30 is a suitable strategy for patients with type 2 diabetes with inadequate glycemic control.
Acknowledgments
The Danish BIAsp study was sponsored by Novo Nordisk A/S and all contributing authors have received support from Novo Nordisk A/S. Statistical analyses were carried out by Novo Nordisk, and all authors contributed to data interpretation and critically reviewed and approved the manuscript, for which writing and editorial assistance was obtained. Editorial assistance in the preparation of this manuscript was provided by Dr. Scott Gouveia and Dr. Catherine Jones of Watermeadow Medical, UK, on behalf of Novo Nordisk A/S. Ann Petruckevitch, Novo Nordisk UK, is thanked for statistical assistance. The Danish BIAsp Study Group includes Marianne Kleis Møller, Charlotte Ørskov, Morten Bech, Birgitte Munk Christensen, Per Warrer Petersen, Lars Hjarnø Knudsen, Kurt Ravnbak, Michael Bjarnhof, Hans Øllgaard, Maj Mortensen, Peter Haase, Per Wium-Andersen, Birgitte Bibow, Henrik Malling Pedersen, Lars Ytte, Niels Holm, Klaus Ruhnau, Tyge Krabbe, Hanne Leufstedt, Henrik Rømer, Ole Tang, Philip Hasselqvist, Christian Raun, Jørgen Hansen, Hans Land Truelsen, Peter Elmegaard, Michel Kjeldsen, Jacob Hassing, Niels Nygaard, Søren Overgaard, Viggo Jensen, Andreas Højring, Kurt Werlinrud, Gert Storm Kristensen, Jan Erik Nielsen, Michael Birch, Connie Bruun Andersen, Ais Egede Jensen, Regnar Rasmussen, Mads Søndergaard, Sven Mehlsen, Jacob Burvil, Anders Evers, Tove Hansen, Jesper Jangaard, Finn Torp, Jens Vang Andersen, Finn Folkenberg, Mette Flarup, Henrik Laursen, Kim Mølenberg, Leif Nielsen, Bent Nielsen, Poul Kraghede, Lone Melgaard Jensen, Lise Storm, Axel Holm, Kaj Folke Hansen, Klaus Roslind, Anders Nørregaard, Jørgen Solgaard, Ejnar Skytte Ankersen, Preben Kjærhus, Niels Steen, Jørn Eisbo, Mogens Mygind, Leif Sehested, Pia Borup, Lars Hagens, Niels Hansen, Bo Gerdes, Jens Eggert, Klaus Nygaard Hansen, Axel Toft, Jørn Ninn-Hansen, Anders Rasmussen, Michael Fløystrup, Christian Schelde, Jacob Olsen, Peter von Scholten, Inger Kristiansen, Erik Heidemann Andersen, Jens Jacobsen, Thorkil Christensen, Jørgen Schmidt Petersen, Jørgen Vinberg, Poul Hven, Lotte Jakobsen, Henrik Sørup Hansen, Helge Rasmussen, Tenna Lauridsen, Peter Tind Ahrenfeldt, Michael Kirkeby Hoffmann, Henrik Ovesen, Lilian Førrisdahl, Bent Drechsler, Lars Faldborg, Erik Mitens, Marianne Mathiesen, Bent Egeberg, Hans-Anton Lenler-Eriksen, Bent Larsen, Henrik Toft, Erik Lassen, Gunnar Axelgaard, Tina Basse, Jens Sandahl Christiansen, Pernille Herman, Per Heden Andersen, Niels Arne Larsen, Nancy Knudsen, Kjeld Hasselström, Kurt Clemmensen, Peter Halkier, Hans-Henrik Steen Darling, Henning Rønne, Eva Ebbehøj, Klaus Michael Pedersen, Jette Vibe-Petersen, Karsten Sølling, Karine Bech, Dorthe Worm, Trine Jensen, Birthe Gade-Rasmussen, Dorthe Hansen, Tonny J. Jensen, Sten Madsbad, Jens Andreassen, Mette Zander Olsen, Grethe Finn Jensen, Bjarne Myrup, Ulla Bjerre Christensen, Anne Jarløv, Annette Svenningsen, Jens Mølvig, Erik Øster-Jørgensen, Kjeld Hermansen.
References
- 1.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 2.Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281(21):2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Standards of medical care in diabetes – 2008. Diabetes Care. 2008;31(Suppl 1):S12–S54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 4.Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006;29(6):1269–1274. doi: 10.2337/dc05-1365. [DOI] [PubMed] [Google Scholar]
- 5.Holman RR, Thorne KI, Farmer AJ, Davies MJ, Keenan JF, Paul S, Levy JC 4-T Study Group. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med. 2007;357(17):1716–1730. doi: 10.1056/NEJMoa075392. [DOI] [PubMed] [Google Scholar]
- 6.Philis-Tsimikas A, Charpentier G, Clauson P, Ravn GM, Roberts VL, Thorsteinsson B. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther. 2006;28(10):1569–1581. doi: 10.1016/j.clinthera.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia. 2008;51(3):408–416. doi: 10.1007/s00125-007-0911-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raskin P, Allen E, Hollander P, Lewin A, Gabbay RA, Hu P, Bode B, Garber A INITIATE Study Group. Initiating insulin therapy in type 2 Diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care. 2005;28(2):260–265. doi: 10.2337/diacare.28.2.260. [DOI] [PubMed] [Google Scholar]
- 9.Kann PH, Wascher T, Zackova V, Moeller J, Medding J, Szocs A, Mokan M, Mrevlje F, Regulski M. Starting insulin therapy in type 2 diabetes: twice-daily biphasic insulin Aspart 30 plus metformin versus once-daily insulin glargine plus glimepiride. Exp Clin Endocrinol Diabetes. 2006;114(9):527–532. doi: 10.1055/s-2006-949655. [DOI] [PubMed] [Google Scholar]
- 10.Weyer C, Heise T, Heinemann L. Insulin aspart in a 30/70 premixed formulation. Pharmacodynamic properties of a rapid-acting insulin analog in stable mixture. Diabetes Care. 1997;20(10):1612–1614. doi: 10.2337/diacare.20.10.1612. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen LV, Søgaard B, Riis A. Pharmacokinetics and pharmacodynamics of a premixed formulation of soluble and protamine-retarded insulin aspart. Eur J Clin Pharmacol. 2000;56(5):399–403. doi: 10.1007/s002280000159. [DOI] [PubMed] [Google Scholar]
- 12.Hermansen K, Colombo M, Storgaard H, Østergaard A, Kølendorf K, Madsbad S. Improved postprandial glycemic control with biphasic insulin aspart relative to biphasic insulin lispro and biphasic human insulin in patients with type 2 diabetes. Diabetes Care. 2002;25(5):883–888. doi: 10.2337/diacare.25.5.883. [DOI] [PubMed] [Google Scholar]
- 13.Boehm BO, Home PD, Behrend C, Kamp NM, Lindholm A. Premixed insulin aspart 30 vs. premixed human insulin 30/70 twice daily: a randomized trial in Type 1 and Type 2 diabetic patients. Diabet Med. 2002;19(5):393–399. doi: 10.1046/j.1464-5491.2002.00733.x. [DOI] [PubMed] [Google Scholar]
- 14.Boehm BO, Vaz JA, Brøndsted L, Home PD. Long-term efficacy and safety of biphasic insulin aspart in patients with type 2 diabetes. Eur J Intern Med. 2004;15(8):496–502. doi: 10.1016/j.ejim.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Garber AJ, Ligthelm R, Christiansen JS, Liebl A. Premixed insulin treatment for type 2 diabetes: analogue or human? Diabetes Obes Metab. 2007;9(5):630–639. doi: 10.1111/j.1463-1326.2006.00654.x. [DOI] [PubMed] [Google Scholar]
- 16.Ligthelm RJ, Borzì V, Gumprecht J, Kawamori R, Wenying Y, Valensi P. Importance of observational studies in clinical practice. Clin Ther. 2007;29(Spec No):1284–1292. [PubMed] [Google Scholar]
- 17.Khutsoane D, Sharma SK, Almustafa M, Jang HC, Azar ST, Danciulescu R, Shestakova M, Ayad NM, Guler S, Bech OM PRESENT Study Group. Biphasic insulin aspart 30 treatment improves glycaemic control in patients with type 2 diabetes in a clinical practice setting: experience from the PRESENT study. Diabetes Obes Metab. 2008;10(3):212–222. doi: 10.1111/j.1463-1326.2007.00826.x. [DOI] [PubMed] [Google Scholar]
- 18.McNally PG, Dean JD, Morris AD, Wilkinson PD, Compion G, Heller SR. Using continuous glucose monitoring to measure the frequency of low glucose values when using biphasic insulin aspart 30 compared with biphasic human insulin 30: a double-blind crossover study in individuals with type 2 diabetes. Diabetes Care. 2007;30(5):1044–1048. doi: 10.2337/dc06-1328. [DOI] [PubMed] [Google Scholar]
- 19.Segel SA, Paramore DS, Cryer PE. Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes. 2002;51(3):724–733. doi: 10.2337/diabetes.51.3.724. [DOI] [PubMed] [Google Scholar]
- 20.Garber AJ, Wahlen J, Wahl T, Bressler P, Braceras R, Allen E, Jain R. Attainment of glycaemic goals in type 2 diabetes with once-, twice-, or thrice-daily dosing with biphasic insulin aspart 70/30 (The 1-2-3 study) Diabetes Obes Metab. 2006;8(1):58–66. doi: 10.1111/j.1463-1326.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- 21.Raskin P, Matfin G, Schwartz SL, Chaykin L, Chu PL, Braceras R, Wynne A. Addition of biphasic insulin aspart 30 to optimized metformin and pioglitazone treatment of type 2 diabetes mellitus: The ACTION Study (Achieving Control Through Insulin Plus Oral ageNts) Diabetes Obes Metab. 2007 doi: 10.1111/j.1463-1326.2007.00796.x. [DOI] [PubMed] [Google Scholar]


