Abstract
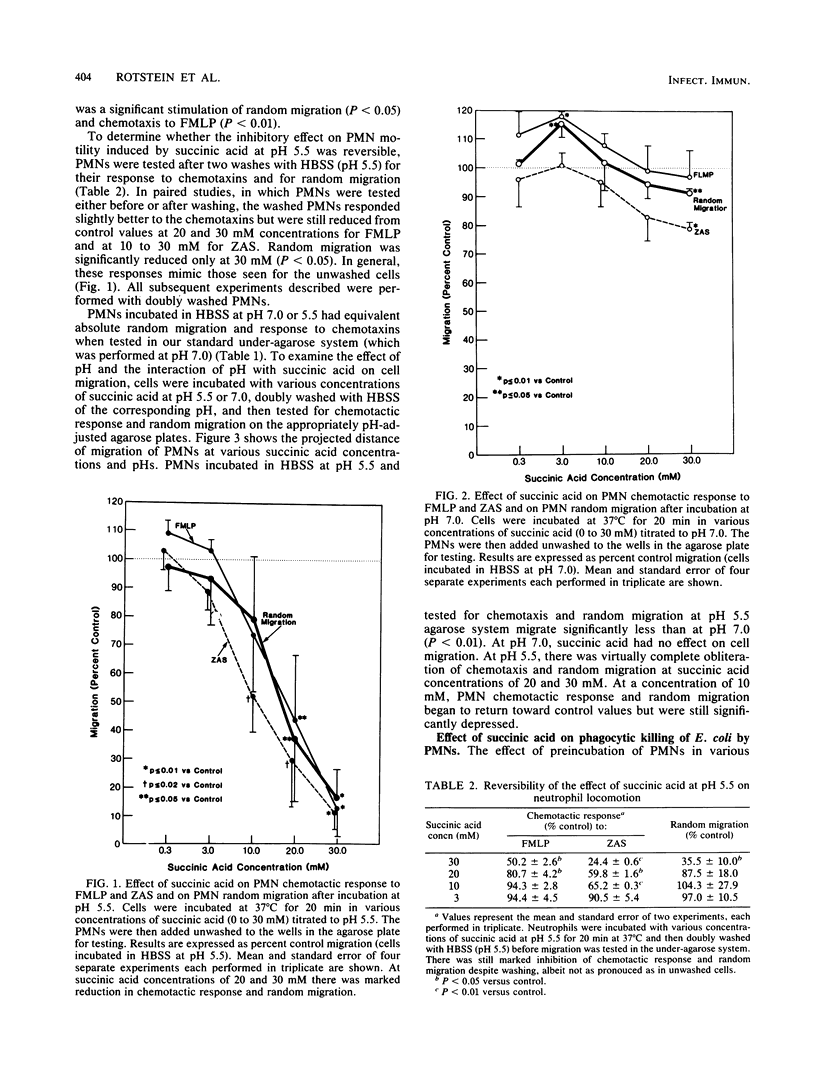
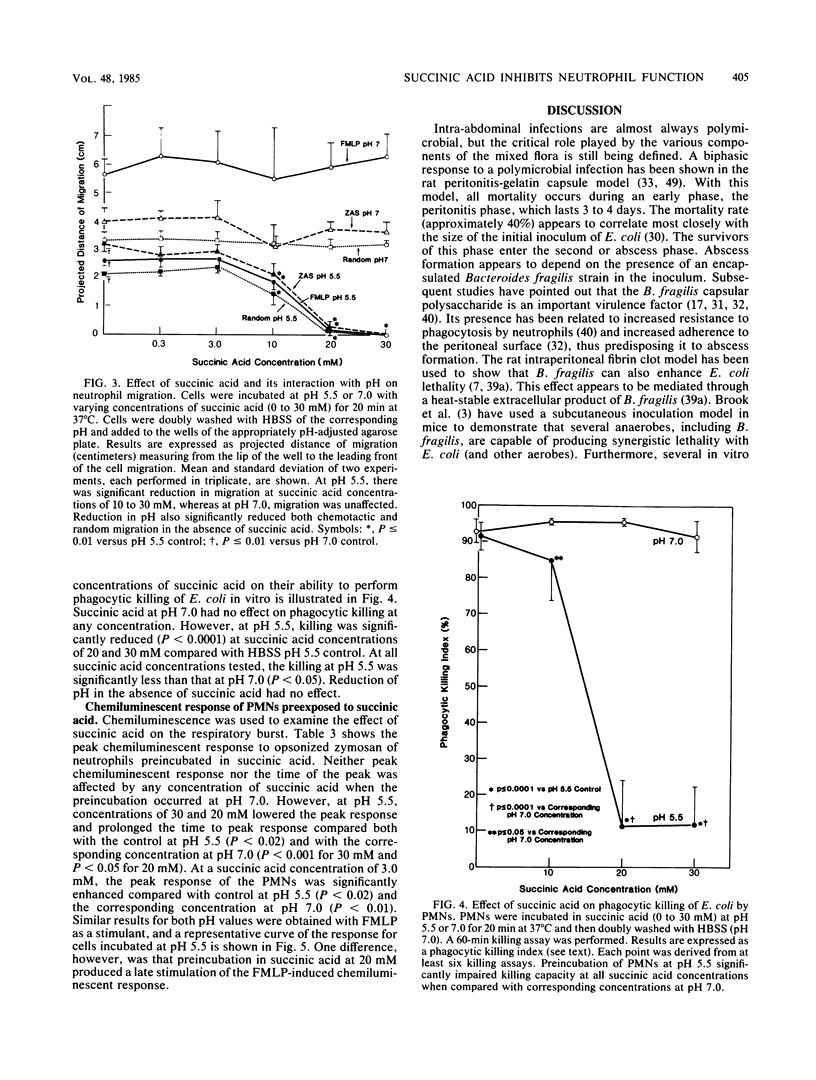
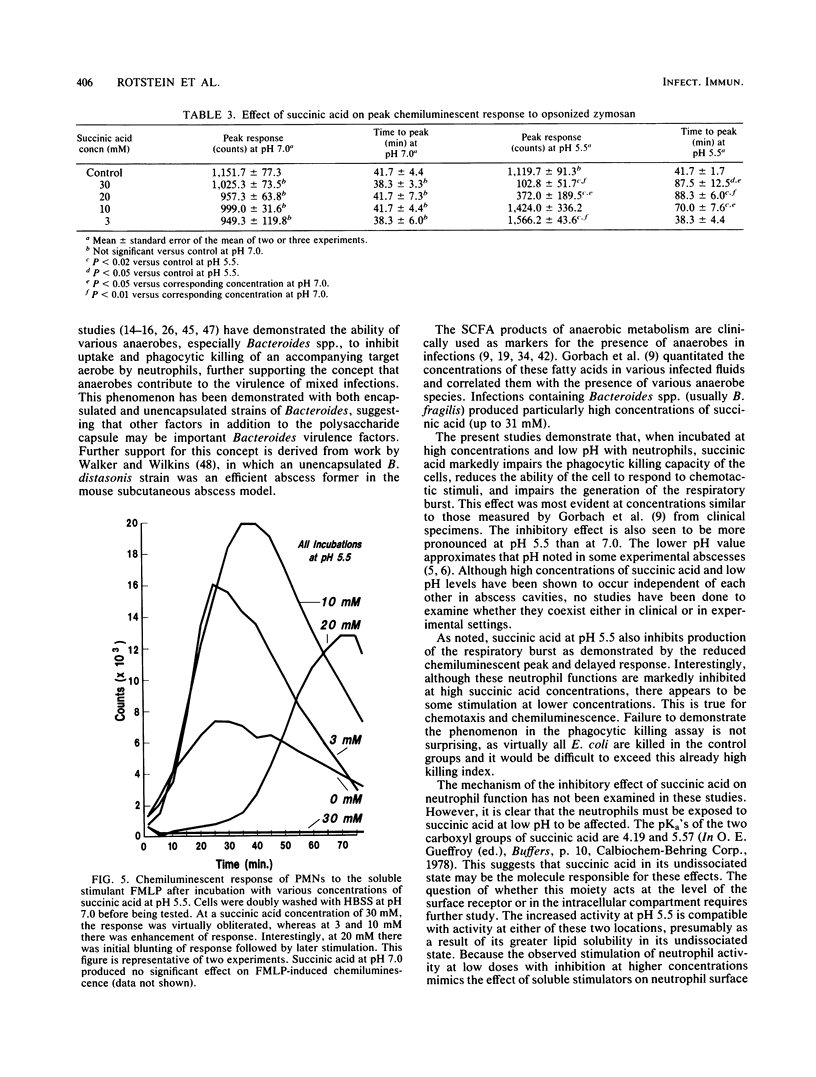
Anaerobes, in particular Bacteroides spp., are the predominant bacteria present in mixed intra-abdominal infections, yet their critical importance in the pathogenicity of these infections is not clearly defined. Succinic acid, a major fatty acid by-product of Bacteroides metabolism, was tested for its effect on neutrophil function to determine whether it might play a role in enhancing the virulence of Bacteroides-containing infections. At pH 5.5 but not pH 7.0, succinic acid at concentrations commonly found in clinical abscesses profoundly inhibits in vitro neutrophil function. It virtually obliterates phagocytic killing of Escherichia coli and reduces neutrophil random migration and chemotactic response to formyl-methionyl-leucyl-phenylalanine and C5a. These effects occur in conjunction with a reduced chemiluminescent peak and delayed time to the peak. The effect on neutrophils is only partially reversible by multiple washings. These findings suggest that succinic acid may be an important Bacteroides virulence factor when present in the microenvironment of a mixed intra-abdominal infection in which concentrations are high and the pH of the medium is reduced.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Loose L. D. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976 Mar 8;69(1):245–252. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- Brook I., Gillmore J. D., Coolbaugh J. C., Walker R. I. Pathogenicity of encapsulated Bacteroides melaninogenicus group, B. oralis and B. ruminicola subsp. brevis in abscesses in mice. J Infect. 1983 Nov;7(3):218–226. doi: 10.1016/s0163-4453(83)97061-5. [DOI] [PubMed] [Google Scholar]
- Brook I., Hunter V., Walker R. I. Synergistic effect of bacteroides, Clostridium, Fusobacterium, anaerobic cocci, and aerobic bacteria on mortality and induction of subcutaneous abscesses in mice. J Infect Dis. 1984 Jun;149(6):924–928. doi: 10.1093/infdis/149.6.924. [DOI] [PubMed] [Google Scholar]
- Brook I., Walker R. I. Significance of encapsulated Bacteroides melaninogenicus and Bacteroides fragilis groups in mixed infections. Infect Immun. 1984 Apr;44(1):12–15. doi: 10.1128/iai.44.1.12-15.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant R. E., Rashad A. L., Mazza J. A., Hammond D. beta-Lactamase activity in human pus. J Infect Dis. 1980 Oct;142(4):594–601. doi: 10.1093/infdis/142.4.594. [DOI] [PubMed] [Google Scholar]
- DUBOS R. J. The micro-environment of inflammation or Metchnikoff revisited. Lancet. 1955 Jul 2;269(6879):1–5. doi: 10.1016/s0140-6736(55)93374-2. [DOI] [PubMed] [Google Scholar]
- Dunn D. L., Rotstein O. D., Simmons R. L. Fibrin in peritonitis. IV. Synergistic intraperitoneal infection caused by Escherichia coli and Bacteroides fragilis within fibrin clots. Arch Surg. 1984 Feb;119(2):139–144. doi: 10.1001/archsurg.1984.01390140005001. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. A rapid one-step procedure for purification of mononuclear and polymorphonuclear leukocytes from human blood using a modification of the Hypaque-Ficoll technique. J Immunol Methods. 1978;24(3-4):389–393. doi: 10.1016/0022-1759(78)90143-6. [DOI] [PubMed] [Google Scholar]
- Gorbach S. L., Mayhew J. W., Bartlett J. G., Thadepalli H., Onderdonk A. B. Rapid diagnosis of anaerobic infections by direct gas-liquid chromatography of clinical speciments. J Clin Invest. 1976 Feb;57(2):478–484. doi: 10.1172/JCI108300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hau T., Hoffman R., Simmons R. L. Mechanisms of the adjuvant effect of hemoglobin in experimental peritonitis. I. In vivo inhibition of peritoneal leukocytosis. Surgery. 1978 Feb;83(2):223–229. [PubMed] [Google Scholar]
- Hau T., Lee J. T., Jr, Simmons R. L. Mechanisms of the adjuvant effect of hemoglobin in experimental peritonitis. IV. The adjuvant effect of hemoglobin in granulocytopenic rats. Surgery. 1981 Feb;89(2):187–191. [PubMed] [Google Scholar]
- Ingham H. R., Sisson P. R., Middleton R. L., Narang H. K., Codd A. A., Selkon J. B. Phagocytosis and killing of bacteria in aerobic and anaerobic conditions. J Med Microbiol. 1981 Nov;14(4):391–399. doi: 10.1099/00222615-14-4-391. [DOI] [PubMed] [Google Scholar]
- Ingham H. R., Sisson P. R., Tharagonnet D., Selkon J. B., Codd A. A. Inhibition of phagocytosis in vitro by obligate anaerobes. Lancet. 1977 Dec 17;2(8051):1252–1254. doi: 10.1016/s0140-6736(77)92662-9. [DOI] [PubMed] [Google Scholar]
- Jones G. R., Gemmell C. G. Impairment by Bacteroides species of opsonisation and phagocytosis of enterobacteria. J Med Microbiol. 1982 Aug;15(3):351–361. doi: 10.1099/00222615-15-3-351. [DOI] [PubMed] [Google Scholar]
- Kasper D. L., Seiler M. W. Immunochemical characterization of the outer membrane complex of Bacteroides fragilis subspecies fragilis. J Infect Dis. 1975 Oct;132(4):440–450. doi: 10.1093/infdis/132.4.440. [DOI] [PubMed] [Google Scholar]
- Kyner D., Zabos P., Christman J., Acs G. Effect of sodium butyrate on lymphocyte activation. J Exp Med. 1976 Dec 1;144(6):1674–1678. doi: 10.1084/jem.144.6.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladas S., Arapakis G., Malamou-Ladas H., Palikaris G., Arseni A. Rapid diagnosis of anaerobic infections by gas-liquid chromatography. J Clin Pathol. 1979 Nov;32(11):1163–1167. doi: 10.1136/jcp.32.11.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. T., Jr, Ahrenholz D. H., Nelson R. D., Simmons R. L. Mechanisms of the adjuvant effect of hemoglobin in experimental peritonitis. V. The significance of the coordinated iron component. Surgery. 1979 Jul;86(1):41–48. [PubMed] [Google Scholar]
- Listgarten M. A. Structure of the microbial flora associated with periodontal health and disease in man. A light and electron microscopic study. J Periodontol. 1976 Jan;47(1):1–18. doi: 10.1902/jop.1976.47.1.1. [DOI] [PubMed] [Google Scholar]
- Lorber B., Swenson R. M. The bacteriology of intra-abdominal infections. Surg Clin North Am. 1975 Dec;55(6):1349–1354. doi: 10.1016/s0039-6109(16)40792-9. [DOI] [PubMed] [Google Scholar]
- MEYNELL G. G. Antibacterial mechanisms of the mouse gut. II. The role of Eh and volatile fatty acids in the normal gut. Br J Exp Pathol. 1963 Apr;44:209–219. [PMC free article] [PubMed] [Google Scholar]
- McCall C. E., Bass D. A., DeChatelet L. R., Link A. S., Jr, Mann M. In vitro responses of human neutrophils to N-formyl-methionyl-leucyl-phenylalanine: correlation with effects of acute bacterial infection. J Infect Dis. 1979 Sep;140(3):277–286. doi: 10.1093/infdis/140.3.277. [DOI] [PubMed] [Google Scholar]
- Namavar F., Verweij A. M., Bal M., van Steenbergen T. J., de Graaff J., MacLaren D. M. Effect of anaerobic bacteria on killing of Proteus mirabilis by human polymorphonuclear leukocytes. Infect Immun. 1983 Jun;40(3):930–935. doi: 10.1128/iai.40.3.930-935.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., Herron M. J., Schmidtke J. R., Simmons R. L. Chemiluminescence response of human leukocytes: influence of medium components on light production. Infect Immun. 1977 Sep;17(3):513–520. doi: 10.1128/iai.17.3.513-520.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., McCormack R. T., Fiegel V. D., Simmons R. L. Chemotactic deactivation of human neutrophils: evidence for nonspecific and specific components. Infect Immun. 1978 Nov;22(2):441–444. doi: 10.1128/iai.22.2.441-444.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Onderdonk A. B., Bartlett J. G., Louie T., Sullivan-Seigler N., Gorbach S. L. Microbial synergy in experimental intra-abdominal abscess. Infect Immun. 1976 Jan;13(1):22–26. doi: 10.1128/iai.13.1.22-26.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk A. B., Kasper D. L., Cisneros R. L., Bartlett J. G. The capsular polysaccharide of Bacteroides fragilis as a virulence factor: comparison of the pathogenic potential of encapsulated and unencapsulated strains. J Infect Dis. 1977 Jul;136(1):82–89. doi: 10.1093/infdis/136.1.82. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Moon N. E., Kasper D. L., Bartlett J. G. Adherence of Bacteroides fragilis in vivo. Infect Immun. 1978 Mar;19(3):1083–1087. doi: 10.1128/iai.19.3.1083-1087.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk A. B., Weinstein W. M., Sullivan N. M., Bartlett J. G., Gorbach S. L. Experimental intra-abdominal abscesses in rats: quantitative bacteriology of infected animals. Infect Immun. 1974 Dec;10(6):1256–1259. doi: 10.1128/iai.10.6.1256-1259.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips K. D., Tearle P. V., Willis A. T. Rapid diagnosis of anaerobic infections by gas-liquid chromatography of clinical material. J Clin Pathol. 1976 May;29(5):428–432. doi: 10.1136/jcp.29.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K. N. Butyric acid: a small fatty acid with diverse biological functions. Life Sci. 1980 Oct 13;27(15):1351–1358. doi: 10.1016/0024-3205(80)90397-5. [DOI] [PubMed] [Google Scholar]
- Prasad K. N., Hsie A. W. Morphologic differentiation of mouse neuroblastoma cells induced in vitro by dibutyryl adenosine 3':5'-cyclic monophosphate. Nat New Biol. 1971 Sep 29;233(39):141–142. doi: 10.1038/newbio233141a0. [DOI] [PubMed] [Google Scholar]
- Rabier D., Cathelineau L., Briand P., Kamoun P. Propionate and succinate effects on acetyl glutamate biosynthesis by rat liver mitochondria. Biochem Biophys Res Commun. 1979 Nov 28;91(2):456–460. doi: 10.1016/0006-291x(79)91543-2. [DOI] [PubMed] [Google Scholar]
- Rabinovich M., DeStefano M. J., Dziezanowski M. A. Neutrophil migration under agarose: stimulation by lowered medium pH and osmolality. J Reticuloendothel Soc. 1980 Feb;27(2):189–200. [PubMed] [Google Scholar]
- Repo H. Leukocyte migration agarose test for the assessment of human neutrophil chemotaxis. I. Effects of environmental factors on neutrophil migration under agarose. Scand J Immunol. 1977;6(3):203–209. doi: 10.1111/j.1365-3083.1977.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Rotstein O. D., Pruett T. L., Simmons R. L. Lethal microbial synergism in intra-abdominal infections. Escherichia coli and Bacteroides fragilis. Arch Surg. 1985 Feb;120(2):146–151. doi: 10.1001/archsurg.1985.01390260016003. [DOI] [PubMed] [Google Scholar]
- Simon G. L., Klempner M. S., Kasper D. L., Gorbach S. L. Alterations in opsonophagocytic killing by neutrophils of Bacteroides fragilis associated with animal and laboratory passage: effect of capsular polysaccharide. J Infect Dis. 1982 Jan;145(1):72–77. doi: 10.1093/infdis/145.1.72. [DOI] [PubMed] [Google Scholar]
- Singer R. E., Buckner B. A. Butyrate and propionate: important components of toxic dental plaque extracts. Infect Immun. 1981 May;32(2):458–463. doi: 10.1128/iai.32.2.458-463.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel C. A., Malangoni M. A., Condon R. E. Gas-liquid chromatography for rapid diagnosis of intra-abdominal infection. Arch Surg. 1984 Jan;119(1):28–32. doi: 10.1001/archsurg.1984.01390130018003. [DOI] [PubMed] [Google Scholar]
- Stone H. H., Kolb L. D., Geheber C. E. Incidence and significance of intraperitoneal anaerobic bacteria. Ann Surg. 1975 May;181(5):705–715. doi: 10.1097/00000658-197505000-00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takazoe I., Tanaka M., Homma T. A pathogenic strain of Bacteroides melaninogenicus. Arch Oral Biol. 1971 Jul;16(7):817–822. doi: 10.1016/0003-9969(71)90126-9. [DOI] [PubMed] [Google Scholar]
- Tofte R. W., Peterson P. K., Schmeling D., Bracke J., Kim Y., Quie P. G. Opsonization of four Bacteroides species: role of the classical complement pathway and immunoglobulin. Infect Immun. 1980 Mar;27(3):784–792. doi: 10.1128/iai.27.3.784-792.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touw J. J., van Steenbergen T. J., De Graaff J. Butyrate: a cytotoxin for Vero cells produced by Bacteroides gingivalis and Bacteroides asaccharolyticus. Antonie Van Leeuwenhoek. 1982;48(4):315–325. doi: 10.1007/BF00418285. [DOI] [PubMed] [Google Scholar]
- Wade B. H., Kasper D. L., Mandell G. L. Interactions of Bacteroides fragilis and phagocytes: studies with whole organisms, purified capsular polysaccharide and clindamycin-treated bacteria. J Antimicrob Chemother. 1983 Oct;12 (Suppl 100):51–62. doi: 10.1093/jac/12.suppl_c.51. [DOI] [PubMed] [Google Scholar]
- Walker C. B., Wilkins T. D. Use of semisolid agar from initiation of pure Bacteroides fragilis infection in mice. Infect Immun. 1976 Sep;14(3):721–725. doi: 10.1128/iai.14.3.721-725.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein W. M., Onderdonk A. B., Bartlett J. G., Gorbach S. L. Experimental intra-abdominal abscesses in rats: development of an experimental model. Infect Immun. 1974 Dec;10(6):1250–1255. doi: 10.1128/iai.10.6.1250-1255.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


