Abstract
The oxidation of H-cluster in gas phase, and in aqueous enzyme phase, has been investigated by means of quantum mechanics (QM) and combined quantum mechanics-molecular mechanics (QM/MM). Several potential reaction pathways (in the above mentioned chemical environments) have been studied, wherein only the aqueous enzyme phase has been found to lead to an inhibited hydroxylated cluster. Specifically, the inhibitory process occurs at the distal iron (Fed) of the catalytic H-cluster (which is also the atom involved in H2 synthesis). The processes involved in the H-cluster oxidative pathways are O2 binding, e- transfer, protonation, and H2O removal.
We found that oxygen binding is non-spontaneous in gas phase, and spontaneous for aqueous enzyme phase where both Fe atoms have oxidation state II; however, it is spontaneous for the partially oxidized and reduced clusters in both phases. Hence, in the protein environment the hydroxylated H-cluster is obtained by means of completely exergonic reaction pathway starting with proton transfer.
A unifying endeavor has been carried out for the purpose of understanding the thermodynamic results vis-à-vis several other performed electronic structural methods, such as frontier molecular orbitals (FMO), natural bond orbital partial charges (NBO), and H-cluster geometrical analysis. An interesting result of the FMO examination (for gas phase) is that an e- is transferred to LUMOα rather than to SOMOβ, which is unexpected because SOMOβ usually resides in a lower energy rather than LUMOα for open-shell clusters.
Keywords: hydrogenase, hydrogenase oxidation, hydrogenase H-cluster, density functional theory, QM/MM calculations, natural bond orbitals, polarized continuum model, frontier molecular orbitals
Introduction
[Fe-Fe]-hydrogenases as well as [Ni-Fe]-hydrogenases are enzymes that are implicated in H2 metabolism (2H+ + 2e- ⇆ H2), which occurs in anaerobic media. Of these two bi-metal enzymes, only [Fe-Fe]-hydrogenases are viable for H2 production, with a reactivity of up to 2 orders of magnitude larger than [Ni-Fe]- hydrogenases 1,2. In hydrogenases, H2 evolution, emerging from proton reduction (2H+ + 2e- → H2), is essential in pyruvate fermentation, and in the disposal of excess electrons. Low-molecular weight biomolecules such as ferredoxins, cytochrome C3, and cytochrome C6 can act as physiological electron acceptors or donors3.
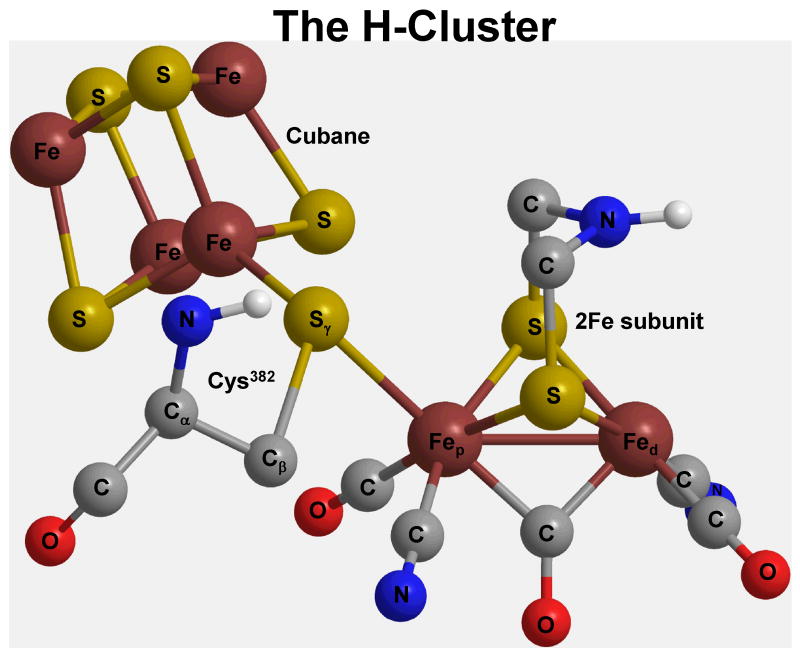
The hydrogenase H-cluster (Scheme 1) is the active site which is comprised of two subunits, the 2Fe subunit, and the cubane, [Fe4-S4]2+, subunit. The 2Fe subunit is composed of two iron atoms (Fep-Fed, i.e., proximal and distal iron) that are bridged by di(thiomethyl)amine (DTMA) chain, and are coordinated by endogenous ligands, i.e., two cyanides, two terminal carbonyls, and a bridging carbonyl (COb). Moreover, the Sγ (of Cys382) is the connecting atom from an Fe atom of the (proximal) cubane subunit and the Fep of the 2Fe subunit.
Scheme 1.
The H-cluster and its subunits, i.e., the cubane, and the 2Fe subunit.
The reason for studying biological H2 production is because the eventual elucidation of the mechanism (for hydrogen synthesis) may benefit researchers produce clean fuel, using certain anaerobic prokaryotes4-8.
Previous Density Functional Theory (DFT) as well as hybrid quantum mechanics/ molecular mechanics (QM/MM) calculations2,9-16 have been successful in clarifying some aspects of the catalytic properties of the H-cluster.
As in similar computational studies2,9, cysteine is substituted with CH3-S-, whereas cubane is replaced* with a H+.
Furthermore, computational and experimental2,9,14,16-41 [Fe-Fe]-hydrogenase H-cluster (and synthetic H-cluster-like compounds) research sheds light on the potential redox states of the 2Fe H-cluster subunit, Fep-Fed, where FepI-FedI is the reduced 2Fe H-cluster subunit, FepII-FedI is the partially oxidized enzyme subunit, and FepII-FedII is the fully oxidized, inactive enzyme H-cluster subunit.
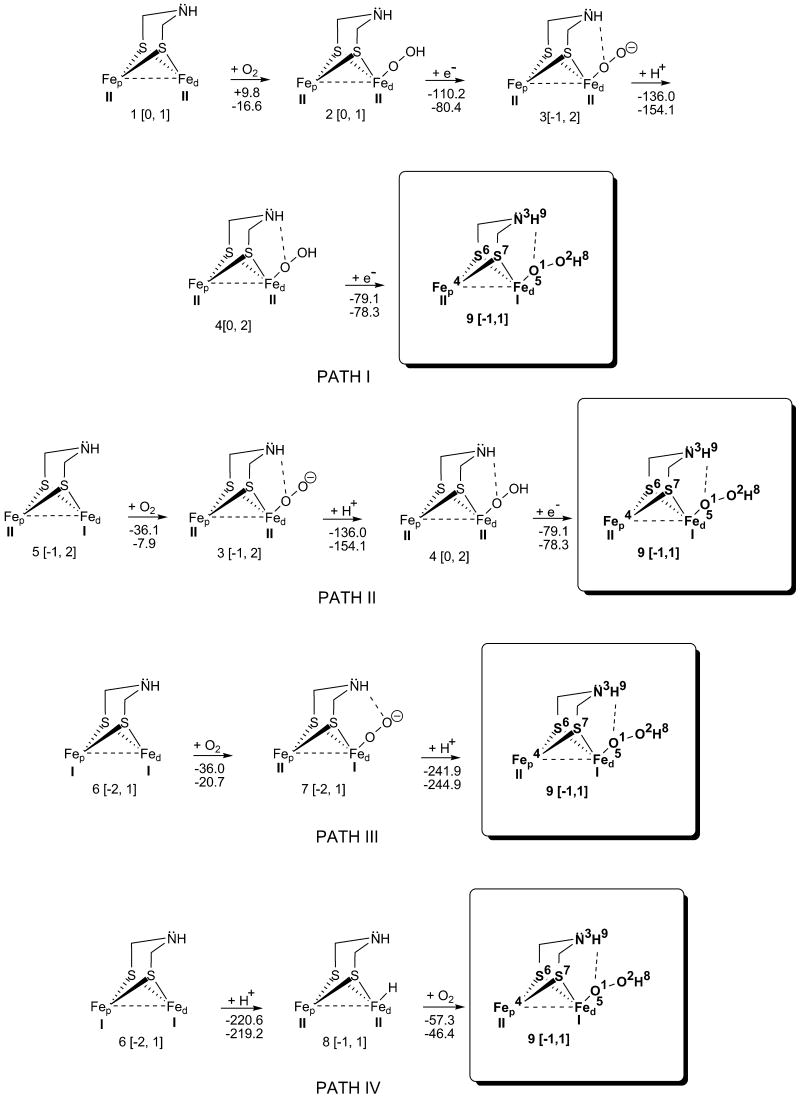
The fully oxidized H-cluster, FepII-FedII, has a H2O molecule or an OH- bound to the FedII. In our previous investigation21, we have inferred that a vacant (fully oxidized) FepII-FedII (1, Figure 1) could also be a viable intermediate in H2 synthesis. Regardless of the 2Fe H-cluster subunit redox states, the proximal cubane [more precisely, a cuboid (point group: D2d)] always retains a 2+ oxidation state, [Fe4-S4]2+.
Figure 1.
Reaction pathways I - IV: Oxidation mechanisms of H-clusters that are fully oxidized (1), partially oxidized (5), and reduced (6). The charges and multiplicities are given in square brackets. The first energy value is for gas phase, and the second is for ONIOM calculations. Fep is the proximal iron, and Fed is the distal iron.
The partially oxidized H-cluster (FepII-FedI, 5, Figure 1), Hox, is the active form of the hydrogenase enzyme†. The reduced H-cluster (FepI-FedI, 6, Figure 1) has both iron atoms in oxidation states I (being an intermediate in H2 metabolism). According to Liu and Hu9, 6 is the cluster having great affinity for protonation (6 → 8), in capturing a proton from the side chain of a near by amino-acid, such as Lys237.
X-ray crystallography and spectroscopic studies of hydrogenases, with the latter having been obtained from Clostridium pasteurianum (CPI)43 and Desulfovibrio desulfuricans (DdH)31, led to a better understanding of the biochemical roles of these enzymes. The X-ray crystal structure of CPI hydrogenase shows an oxygen species that may be OH-, or H2O bound to the Fed of the H-cluster. Based on the computational results of Liu and Hu2 (CPI has OH- in its inactive form according to X-ray crystal structure), we endeavor to ascertain whether the air oxidized H-cluster (Fep-Fed-O2) converts to Fep-Fed-OH species21.
Our investigation is composed of three different subdivisions. 1). Thermodynamic analysis, for every reaction path mechanism (Figures 1 - 3), implicated in the eventual H-cluster 14 inhibition by means of O2 → OH-. 2). Electronic analysis, for the same paths, which deals with Natural Bond Orbitals (NBO), as well as Frontier Molecular Orbitals (FMO). 3). Geometrical analysis carried out only for appropriate bond breaking, and bond formation.
Figure 3.
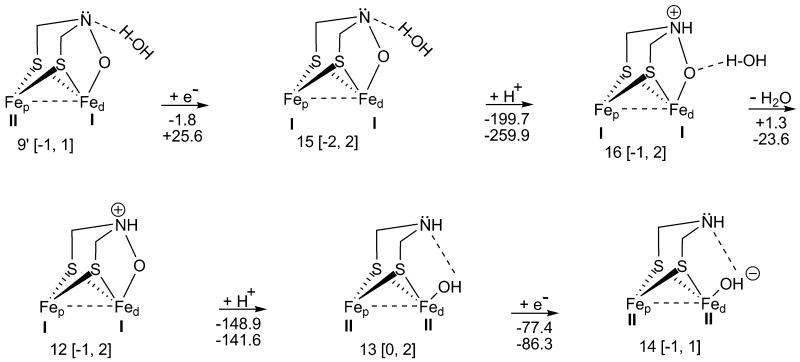
Reaction mechanism for reduction, protonation, and H2O removal from the inhibited [Fe-Fe]-hydrogenase H-cluster. Here, the H2O is being eliminated from an open-shell cluster. (The charges, multiplicities, and energy values are presented as in Figure 1.)
From the investigated subdivisions, thermodynamics analysis (Figure 2, and 3) is of pivotal importance since it shows that there is just an exergonic path from H-cluster 9′ to the hydroxylated cluster 14 occurring in the aqueous enzyme phase. However, from the thermodynamic results (Figure 1), it is observed that most reaction steps proceed exergonically (except 1 → 2, gas phase), leading to the oxidized cluster 9. Moreover, at the end of each path, every vacant H-cluster 1, 5, and 6, in spite of its oxidation states, becomes aerobically inactivated, 9.
Figure 2.
Reaction mechanism for isomerization, protonation, H2O elimination, and reduction of the inhibited [Fe-Fe]-hydrogenase H-cluster. The H2O is being removed from a closed-shell cluster. (The charges, multiplicities, and energy values are presented as in Figure 1.)
Thus, this paper is organized as follows: in the Methods we describe the QM/MM partitioning, and the methods used for calculations (e.g., QM Hamiltonian, basis sets, and the force field parameters). Then, in the Results and Discussion, we present the computational results organized into subsections that present and discuss thermodynamic results, geometrical, and electronic data for different steps of the reaction pathways, such as oxygen binding, reduction, oxidation, and water elimination. Finally, in the Conclusion we give a summary of our findings.
Methods
In the current study, both QM [DFT (in gas phase)] and QM/MM [DFT/UFF44 (in aqueous enzyme phase)] methodologies have been used. The ONIOM45 method (DFT for the QM region, and the universal force field, UFF, for the MM region, implemented in Gaussian0346) has been applied to determine the reaction thermodynamics, i.e., ΔG, for the inactivation pathways of the H-cluster, and the [Fe-Fe]-hydrogenase H-cluster (positioned within the enzyme matrix). Subsequently, the DFT results have been compared with the ONIOM calculations. The electronic structure of the hydrogenase active site (except the proximal cubane) is investigated by quantum mechanics (Gaussian 03) using DFT method (B3LYP functional47,48), and QM/MM with 6-31+G(d,p) basis set. For Fe an effective-core potential with a double zeta polarization basis set (LANL2DZ49,50) was used for DFT gas phase calculations, and a 6-31+G(d,p) basis set for the ONIOM calculations. In accordance with experimental and in-silico data low spin states (singlet, and doublet), and low oxidation states (I, and II) have been selected for the Fe atoms2,14,35. Gromacs program51,52 was employed to add hydrogen atoms, water, and counter ions to the X-ray crystal structure of DdH [Brookhaven Protein Data Bank id.1HFE]. Hydrogen atoms and a 1 nm layer of water (2043 molecules) have been added to the PDB DdH structure. Moreover, Na+ ions have been randomly inserted into the solvent to neutralize the negative charges encountered therein, e.g., the -2 a.u. found on the cubane/cysteine moieties‡53. For both basic and acidic amino acids, charges were assigned by Gromacs algorithm to be at pH 7. ONIOM geometry optimizations have been performed on the DdH, with the low layer (MM region) being frozen*, with the exception of the proximal cubane, while the high layer (QM) had only the iron atoms, Fep-Fed, and the N3, (of the DTMA bridge) kept frozen; “freezing atoms” is practiced to reduce computational time. The low layer consists of all the hydrogenase amino acids as well as its constituent cubanes, i.e., proximal, medial, and distal. The high layer is comprised of 2Fe subunit, (which is the moiety of the H-cluster), and Cβ and Sγ (appertaining to the bridging Cys382). Moreover, two linking hydrogen atoms were added between Cα and Cβ of Cys382, and between Sγ and an Fe atom of the proximal cubane. The charge equilibration method of the UFF was used to describe the electrostatic interactions within the low layer of the system54. The DdH partial charges were obtained using the charge equilibration method, whereas the solvent charges were acquired from literature54 (qO = -0.706 a.u. and qH = 0.353 a.u.).
Results and Discussion
H-cluster Thermodynamics for O2 Binding, Reduction, and Protonation
Figure 1 illustrates different O2 inhibition pathways of the hydrogenase H-cluster; the H-clusters, 1, 5, 6, and 82,9,21, of the pathways are obtained in the reversible catalysis of H2. Reaction 1 → 2 (path I) is endergonic for the gas phase (ΔGgas = +9.8 kcal/mol; gas = gas phase) when O2 binds to the fully oxidized H-cluster (1). ONIOM calculations, on the other hand, show that O2 binding occurs exergonically (ΔGQM/MM = -16.6 kcal/mol), shedding light on the sensitivity of hydrogenases to O255.
Reduction 2 → 3 (ΔGgas = -110.2 kcal/mol) as well as protonation 3 → 4 (ΔGgas = -136.0 kcal/mol) proceed exergonically; ONIOM calculations, for the hydrogenase matrix, show that e- transfer is considerably less exergonic (ΔGQM/MM = -80.4 kcal/mol) relative to protonation which is more exergonic (ΔGQM/MM = -154.1 kcal/mol). The free energy differences, in gas vs. aqueous enzyme phases for reactions 2 → 3 and 3 → 4, ensue from the effect of the electric field of the protein on the H-clusters 2, 3, and 4, and from the different phase geometries.
Cluster 4 undergoes reduction, and it (4 → 9) proceeds exergonically in both gas and aqueous hydrogenase phase (ΔGgas = -79.1 kcal/mol; ΔGQM/MM = -78.3 kcal/mol).
Path II starts with the partially oxidized H-cluster 5, (FepII-FedI). The binding of O2 to FeI (FedI-O2), 5 → 3, is firmer (ΔGgas = -36.1 kcal/mol) than for FeII in 1 → 2 (FedII-O2, path I). In contrast, ONIOM results show that O2 binds to the partially oxidized H-cluster (FepII-FedI, ΔGQM/MM = -7.9 kcal/mol) as well as to the fully oxidized cluster (FepII-FedII, ΔGQM/MM = -16.6 kcal/mol, 1 → 2, path I). The remaining two reactions 3 → 4, and 4 → 9 (path II) are the same as the last two steps of path I.
In path III, 6 → 7, which starts with the fully reduced H-cluster 6, (FepI-FedI), the reaction spontaneity (ΔGgas = -36.0 kcal/mol) is almost identical to the free energies of reaction 5 → 3. The gas phase free energy similarity may ensue because both loci of oxygen binding (FedI-O2) are on similar oxidized species, FedI. However, ONIOM calculations show smaller reaction spontaneity difference between aqueous enzyme (ΔGQM/MM = -20.7 kcal/mol) and gas phase results (ΔGgas = -36.0 kcal/mol, 6 → 7), than for O2 binding in path I and II. In path III, protonation (7 → 9) is, once again, largely exergonic for both phases (ΔGgas = -241.9 kcal/mol; ΔGQM/MM = -244.9 kcal/mol). Moreover, from all of Figure 1, the above ONIOM calculations show the highest H+ affinity because H-cluster 7 has a charge of -2 a.u., and also the H+ binds to a rather electronegative atom, viz., oxygen.
In the final path (IV), protonation 6 → 8 is the second most exergonic reaction in gas phase (ΔGgas = -220.6 kcal/mol) mostly because of the over-all charge of -2 on the H-cluster 6. ONIOM data (as in 7 → 9) show very high H+ affinity (ΔGQM/MM = -219.2 kcal/mol) for the hydrogenase H-cluster (in spite of the fact that the H+ is seized by the Fed as opposed to the more electronegative Fed-O2, 7 → 9), which is comparatively similar to the gas phase result (ΔGgas = -220.6 kcal/mol).
In the last step (8 → 9; path IV), O2 is interposed between Fed and the hydride (FedI-O2-H, 9). For this insertion reaction, the O2 binding occurs exergonically in both ONIOM (ΔGQM/MM = -46.4 kcal/mol) and the gas phase (ΔGgas = -57.3 kcal/mol) results.
N.B., path IV shows that oxidation of Fep-Fed H-cluster is similar* to the Nip-Fed hydrogenase H-cluster obtained from experimental data56.
From the above thermodynamic results, most reaction steps proceed exergonically (except 1 → 2, gas phase), leading to oxidized cluster 9. At the end of every path, each vacant H-cluster 1, 5, or 6, in spite of its oxidation states, becomes aerobically inactivated.
NBO Charges and Geometry Adjustment of Intermediates in the Oxidation of H-cluster
The atoms of the vacant H-clusters 1, 5, and 6 have slightly different natural bond orbital (NBO) charge distributions. For instance, for cluster 1 the NBO charges of Fep-Fed are qgFep** = 0.137 a.u. (qeFep = -0.230 a.u.) and qgFed = -0.096 a.u. (qeFed = 0.187 a.u.), whereas in 5, the sign of the partial charges are reversed only in gas phase, i.e. qgFep = -0.024 a.u. (qeFep = -0.227 a.u.) and qgFed = 0.078 a.u. (qeFed = 0.061 a.u.). Then, the NBO charges for the Fep-Fed in cluster 6 (in both phases) are more negative, qgFep = -0.104 a.u. (qeFep = -0.297 a.u.), and qgFed = -0.117 a.u. (qeFed = -0.160 a.u.) because both metals are in a reduced state (Figure 1), unlike clusters 1 and 5. Regarding charges on the nitrogen, N3, (of the DTMA bridge), similarities are seen amongst clusters 1, 5, and 6; the NBO charges for N3 are approximately -0.700 a.u., making this amine (within the above H-clusters) a relatively important H+ acceptor/donor (vs. amino acids with similar function in the juxtaposed enzyme matrix, e.g., Lys237) as suggested by Liu and Hu9. The non-bridging Sγ (of Cys382) has the following charges: for 1 qgSγ = 0.204 a.u. (qeSγ = 0.474 a.u.), for 5 qgSγ = 0.142 a.u. (qeSγ = 0.425 a.u.), and for 6 qgSγ = 0.079 a.u. (qeSγ = 0.285 a.u.). Comparing clusters 1, 5 and 6, a sequential drop in NBO charges for Fep and Sγ is observed.
When H-cluster 1 is in an oxidized state (in gas phase), FepII- FedII, the COb shifts9 towards the FedII, and becomes bonded to FedII. The shifted COb bond distance (measured from its bridging carbon, Cb, to the iron atoms) between Cb—FepII is 3.067 Å, whereas Cb—FedII is 1.819 Å. When the carbonyl is close to FedII, COb-FedII, the fully oxidized H-cluster 1 becomes relatively stable vs. the quasi-symmetric cluster21 (ΔH = 14 kcal/mol), which is also shown by the NBO charge on Cb in COb, (0.664 a.u.). This may be due to repulsion of charges between qgCb (0.664 a.u.) and qgFep (0.137 a.u.), whereas for the clusters 5 and 6, the partial charges (qgCb = 0.462 a.u., and qgCb = 0.466 a.u., respectively) are less then in 1 because COb is bonded to both iron atoms. However, in the enzyme phase less shifting of the bridging carbonyl occurs, with the charges on Cb being similar (qeCb = 0.536 a.u. for 1, qeCb = 0.497 a.u. for 5, and qeCb = 0.493 a.u. for 6). Comparing the reaction spontaneity for O2 binding (in gas phase), which renders clusters 2, 3, and 7, one may observe that 3 and 7 are more stable than 2. The reason for this stability is essentially due to the formation of a hydrogen bond between the exogenous O2 and the hydrogen H9 (bonded to N3 of the DTMA bridge) in both 3 and 7. The O1—H9 bond distance is 2.016 Å in 3, and O2—H9 bond distance is 1.765 Å in 7, which correlates with the NBO charges on the mentioned oxygens and hydrogens; the partial charges on oxygens are qgO1 = -0.235 a.u. in 3, and qgO2 = -0.493 a.u. in 7, whereas the charge on H9 is 0.439 a.u. in 3, and 0.454 a.u. in 7. Note that COb is located almost symmetrically in clusters 2, 3, and 7. Structurally, clusters 4 and 9 are similar in view of the fact that both possess a hydrogen bond (H9…O1), whereas COb is found to reside quasi-symmetrically in 9, but asymmetrically in 4 bonded only to FepII. In the enzyme phase both hydrogen bonds (in clusters 3 and 7) are formed between H9 and O2 of the exogenous oxygen (qeO2 = -0.210 a.u. in 3, qeO2 = -0.452 a.u. in 7, qeH9 = 0.443 a.u. in 3, and qeH9 = 0.445 a.u. in 7; O2—H9 bond distance is 1.890 Å in 3, and O2—H9 bond distance is 1.760 Å in 7).
Thermodynamics and NBO charges relationship for H2O Removal from the Oxidized H-cluster
Figure 2 depicts a series of reactions (9 → 9′, 9′ → 10, 10 → 11, 11 → 12, 12 → 13, and 13 → 14), which present the net conversion of 9 to 14. The compounds 9 and 9′ are isomers; 9′ is more stable by 47.2 kcal/mol, [which may be, to a certain extent, attributed to the hydrogen bond formation between H2O and the N3 of DTMA bridge (N3…H-OH; i.e., 2 bonds being broken vs. 3 being formed for 9 → 9′, respectively)]. The hydrogen bond length, N3…H, is 1.939 Å (and the angle formed by N3…H-O is 168.7°). The distance between the iron atoms is larger in 9′ (2.796 Å) than in 9 (2.605 Å). During reaction 9 → 9′, COb moves away from FepII [i.e., for Cb-FepII 2.225 Å (9) → 2.771 Å (9′)]. Also, in Figure 2 (hydrogenase H-cluster 9, and 9′), ONIOM geometry optimizations for 9, and 9′ resulted in the same structure for the hydrogenase H-clusters (ΔGQM/MM corresponding to 9 → 9′ is 0 kcal/mol). The protonation of 9′ (9′ → 10) produces a quaternary ammonium ion (NR4+) within the DTMA bridge, which is exergonic for both phases, viz., ΔGgas = -130.7 kcal/mol, and ΔGQM/MM = -138.4 kcal/mol. To wit, the observed high reaction spontaneity for both phases is attributed to the negatively charged H-cluster 9′. In 10 → 11, H2O is removed from N3 by means of hydrogen bond breaking; this reaction (vs. 9′ → 10) occurs slightly endergonically in gas phase (ΔGgas = +3.3 kcal/mol), while for QM/MM results, the H2O removal step, 10 → 11, is exergonic (ΔGQM/MM = -24.1 kcal/mol).
Reduction 11 → 12 (Figure 2) is subjected to an increase in the partial charge of the exogenous oxygen (qgO1 = -0.568 a.u. (11) → -0.594 a.u. (12); ΔGgas = -72.8 kcal/mol). Regarding geometrical changes in 11 → 12, the bond distance between FepII-FedI is increasing from 2.792 Å to 3.261 Å, while the COb departs from FepII [for Cb-FepII 2.766 Å (11) → 3.183 Å (12)]. For the aqueous enzyme phase result, 11 → 12 occurs with a relatively large free energy (ΔGQM/MM = -95.4 kcal/mol; compared to other neutral H-cluster reductions), versus the gas phase outcome (ΔGgas = -72.8 kcal/mol); the charge remains constant on the exogenous oxygen [qeO1 = -0.530 a.u. (11) → -0.527 a.u. (12)]. Due to excess electron density accumulation on O1 (12), the latter readily captures a proton (12 → 13; ΔGgas = -148.9 kcal/mol). ONIOM calculations, 12 → 13, confirm the high H+ affinity (in Figure 2, ΔGQM/MM = -141.6 kcal/mol) for the hydrogenase H-cluster, which is close to the gas phase result (ΔGgas = -148.9 kcal/mol). The free energy differences between the given protonations, 12 → 13 vs. 9′ → 10, may arise because of the greater stability of cluster 13 vs.10.
Finally, in Figure 2, an e- is acquired by the hydroxyl group (13 → 14; ΔGgas = -77.4 kcal/mol; ΔGQM/MM = -86.3 kcal/mol). Note that the H-cluster 142,21 is the starting compound in the reactivation pathway that ends in the reduced H-cluster 6 (FepI-FedI).
In Figure 3, an alternative pathway (9′ → 15, 15 → 16, 16 → 12, 12 → 13, and 13 → 14) has been investigated. The pathway starts with a reductive step, rather than with a protonation. Reaction 9′ → 15 is slightly exergonic for the gas phase (ΔGgas = -1.8 kcal/mol), while ONIOM calculations indicate an endergonic process (ΔGQM/MM = +25.6 kcal/mol). 9′ → 15 is another O2 inhibitory step (in addition to 10 → 11 for the gas phase, Figure 2) which seems to explain the O2 sensitivity of wild type DdH. Therefore, mutagenic studies ought to be performed on [Fe-Fe]-hydrogenase H-cluster 9′ to eliminate its inhibitory path (viz., 9′ → 15). When a H+ is in the vicinity of H-cluster 15, 15 → 16 proceeds with the greatest spontaneity (of Figures 2 and 3) in gas phase (ΔGgas = -199.7 kcal/mol) because 15 has a net charge of -2 a.u. Note that the ONIOM findings, for step 15 → 16, confirm the highest free energy (ΔGQM/MM = -259.9 kcal/mol) of all the potential reaction mechanisms analyzed for the [Fe-Fe]-hydrogenase H-cluster, while the gas phase result is about 60 kcal/mol less exergonic. Water elimination in gas phase, (16 → 12) is slightly endergonic (ΔGgas = +1.3 kcal/mol), whereas for the aqueous enzyme phase it is significantly exergonic (ΔG QM/MM = -23.6 kcal/mol). Note that both 10 (Figure 2) and 16 (Figure 3) lead to the same compound (12) by H2O elimination. The thermodynamic data are similar for both reactions, 10 → 11 and 16 → 12, because Fed is found in the same oxidation state (FedI) in both 10 and 16, but Fep (being further away from the focal catalytic locus, Fed) has different oxidation states [FepII (10); FepI (16)]. The in silico ONIOM result of the H2O removal step (Figure 3, 16 → 12), is exergonic (ΔGQM/MM = -23.6 kcal/mol), just like in step 10 → 11, (Figure 2, ΔGQM/MM = -24.1 kcal/mol). Also, close free energies are observed for the gas phases of 16 → 12 (ΔGgas = +1.3 kcal/mol) and 10 → 11 (ΔGgas = +3.3 kcal/mol). Next, reactions 12 → 13, and 13 → 14 proceed exergonically [(ΔGgas = -148.9 kcal/mol; ΔGQM/MM = -141.6 kcal/mol), and (ΔGgas = -77.4 kcal/mol; ΔGQM/MM = -86.3 kcal/mol), respectively], just as (previously discussed) in Figure 2. The following reactions, 10 → 11 (Figure 2) and 16 → 12 (Figure 3), show that the entire (oxidative inhibitory H-cluster) path has difficulties proceeding to 14 in gas phase.
From the above, it can be seen that there is only one exergonic path (Figure 2) from the oxidized H-cluster 9′ to the hydroxylated cluster 14 in aqueous enzyme phase. A path starts with H+ transfer (Figure 2), while the other begins by e- transfer (Figure 3). The gas phase H2O elimination, from the oxidized H-cluster, proceeds endergonically in both pathways (Figure 2 and 3).
Frontier Molecular Orbital Analysis
Electronic contributions are now presented for both phases, which are adduced by the frontier molecular orbitals in conjunction with the previously presented free energies.
Upon reduction of open-shell H-clusters, it is observed that an e- is obtained by a semi-occupied molecular orbital (SOMO), while the closed-shell clusters receive an e- into the lowest virtual molecular orbital (LUMO). However, when a H+ is in the proximity of an open-shell H-cluster, it can form a σ-bond probably through the interaction of the e- in the highest occupied molecular orbital (HOMO), or through the contributions of both HOMO and SOMO, with the proviso that the SOMO is sufficiently low in energy relative to HOMO. Alternatively, when a H+ is near a closed-shell cluster, the σ-bond probably ensues mainly due to the contribution of e-s from HOMO with the H+.
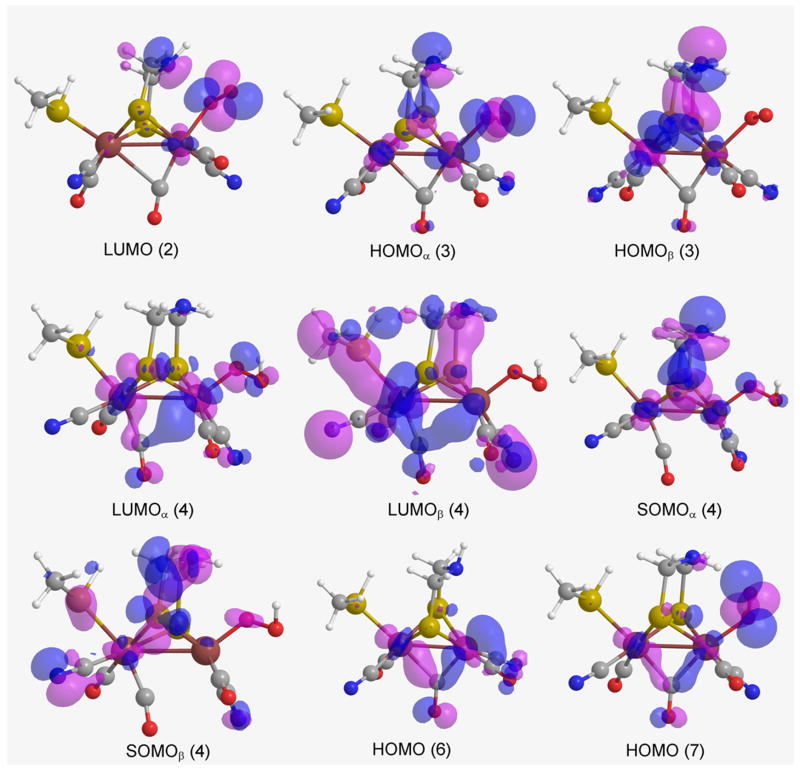
Gas phase thermodynamic properties, of the reactions in Figs. 1, 2, and 3, are being examined with regard to frontier molecular orbitals (FMO). Thus, in 2 the LUMO (Figure 4) is mostly localized on the exogenous O2 and N3, which is also corroborated by an increase of NBO charges on O2 and N3 in 3 upon reduction of H-cluster 2 [qgO1 = -0.046 a.u. (2) → -0.235 a.u. (3); qgN3 = -0.568 a.u. (2) → -0.717 a.u. (3)].
Figure 4.
Frontier molecular orbitals (gas phase) for H-clusters LUMO (2), HOMOα (3), HOMOβ (3), LUMOα (4), LUMOβ (4), SOMOα (4), SOMOβ (4), HOMO (6), and HOMO (7) (where the atom colors, of the H-clusters, are O = red, C = grey, N = blue, S = yellow, Fe = burgundy, and H = white).
Aqueous enzyme phase thermodynamic properties are next being examined for the reactions of Figs. 1, 2, and 3 relative to the frontier molecular orbitals (FMO).
For 2, LUMO [Figure 6, (compare to Figure 4 in gas phase)] is mostly localized on Sγ of Cys382 (as opposed to cluster 2 in gas phase) owing to the electronic contribution of the proximal cubane. Additionally, the localization of LUMO is supported by a decrease of NBO charge on Sγ in 3 upon reduction of H-cluster 2 [qeSγ = 0.471 a.u. (2) → 0.388 a.u. (3)].
Figure 6.
Frontier molecular orbitals (aqueous enzyme phase) for H-clusters LUMO (2), HOMOα (3), HOMOβ (3), LUMOα (4), LUMOβ (4), SOMOα (4), SOMOβ (4), HOMO (6), and HOMO (7).
For open-shell clusters, unrestricted B3LYP calculations have been performed which resulted in different quantum mechanical (QM) energies and molecular orbital (MO) coefficients for α and β electrons.
In gas phase, the HOMOα (the lower energy HOMO containing a spin up e-) of 3 is predominantly localized on the exogenous O2, where the protonation also occurs. However, the HOMOβ (the higher energy HOMO with its spin down e-) is localized on the DTMA bridge (Figure 4).
For the aqueous enzyme phase, the HOMOα of 3 is less localized on the exogenous O2 (relative to the gas phase situation), but this orbital is essentially localized on the DTMA bridge.
The HOMOβ, relative to the gas phase electronic distribution, is more localized on the exogenous O2 (Figure 6), supporting the greater spontaneity of H+ transfer (3 → 4).
The SOMOα of compound 4, in gas phase, is mostly localized on the DTMA bridge, and, to some extent, on the exogenous O2 and the Fe atoms (Figure 4). SOMOβ is more delocalized than SOMOα. Following the e- transfer 4 → 9, the main change in partial charges occurs on the iron atoms [qgFep = -0.141 a.u. (4) → -0.003 a.u. (9); qgFed = 0.464 a.u. (4) → 0.025 a.u. (9)]. The change in NBO charges in 4 → 9 can be corroborated by LUMOα (4; Figure 4). It is noteworthy that the e- is transferred into the LUMOα (-0.15381 Hartrees), for its energy is lower than that of SOMOβ (-0.14425 Hartrees). For the hydrogenase, LUMOα (4, Figure 6) is also lower in energy than SOMOβ (4, Figure 6) (ELUMOαEnzyme = -0.35944 Hartrees, ESOMOβEnzyme = -0.35907 Hartrees), implying that the e- is transferred to and localized on Sγ of Cys382. However, this difference in electron localization is not reflected in the reaction thermodynamics, because 4 → 9 is similarily exergonic in both phases.
The HOMO of 6, in gas phase, is localized on the Fed and the COb, whereas the HOMO of 7 is primarily localized on the exogenous O2 but is less diffused over COb (Figure 4). The proton binds with high affinity to Fed of H-clusters 6 (Path IV) and 7 (Path III) because the HOMO orbitals of these clusters are localized on Fed and exogenous O2, respectively. In particular, 7 manifestly displays where protonation occurs, viz., on the exogenous O2 (Figure 1).
In aqueous enzyme phase, similar electron orbital distributions are encountered for clusters 6 (Path IV) and 7 (Path III), except that Sγ (of Cys382) incurs MO distributions, which may be sustained by the proximal cubane (that facilitates the e- transfer).
The HOMO of 9′, is delocalized throughout the cluster and, has smaller proton affinity in comparison to 6 and 7. However, higher HOMO 9′ amplitude is found on the exogenous O1, DTMA bridge, and the two irons which may explain why the N3 is being protonated in this case.
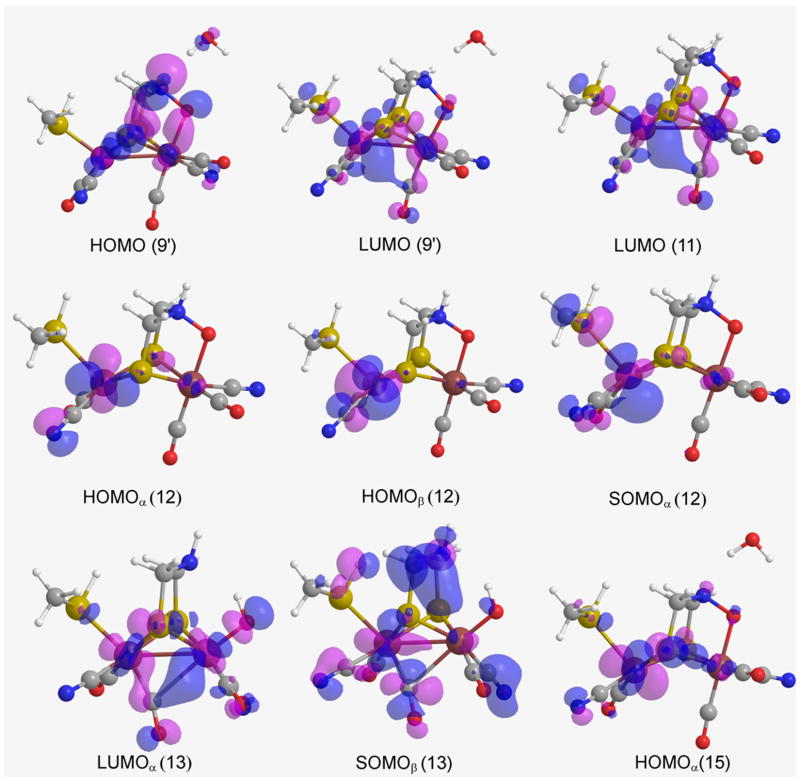
For cluster 11, the LUMO is more localized over the Fep than on Fed, extending from the irons towards the COb via a linear combination between the eg orbitals of the iron atoms with the COb π orbitals9, thus the e- transfer, 11 → 12, changes the oxidation state of Fep. However, for ONIOM, the LUMO is localized on Sγ which is being bereft of e-s via an inductive effect of the vicinal cubanes [qeSγ = 0.464 a.u. (11) vs. 0.333 a.u. (12)].
Both HOMOα and HOMOβ, of 12, are generally localized on the Fep (Figure 5). However, in this case, the protonation does not occur at the Fed, instead it occurs at the exogenous O1 since its NBO charge is very negative, i.e., qgO1 = -0.594 a.u. as opposed to qgFed = 0.126 a.u. On the other hand, for the aqueous enzyme phase, both HOMOα and HOMOβ, of 12, differ in their distribution, especially HOMOα having orbital amplitude on the exogenous oxygen, making it a good H+ acceptor.
Figure 5.
Frontier molecular orbitals (gas phase) for H-clusters HOMO (9′), LUMO (9′), LUMO (11), HOMOα (12), HOMOβ (12), SOMOα (12), LUMOα (13), SOMOβ (13), and HOMOα (15).
Cluster 13 is an open-shell cluster, so upon its reduction an e- may either enter a LUMOα, or a SOMOβ depending on their relative orbital energies. In the reductive process of H-cluster 13 (for gas phase), the in silico data explicitly shows that the orbital energy of LUMOα (ELUMOαgas = -0.14850 Hartrees) is lower than the energy of SOMOβ (ESOMOβgas = -0.13886 Hartrees). Nevertheless, these energies are almost identical in the aqueous enzyme phase (ELUMOαenzyme = -0.35177 Hartrees, ESOMOβenzyme = -0.35185 Hartrees). Thus, upon reduction of cluster 13, the e- could enter into LUMOα (Figures 5 and 7 of both phases). Upon analysis of the NBO charges of clusters 13 and 14, the OH- and Fed of 14 acquire most of the partial charge ceded by Fep during the reductive process 13 → 14.
Figure 7.
Frontier molecular orbitals (aqueous enzyme phase) for H-clusters HOMO (9), LUMO (9), LUMO (11), HOMOα (12), HOMOβ (12), SOMOα (12), LUMOα (13), SOMOβ (13), and HOMOα (15).
Finally, in gas phase H-cluster 15 undergoes a protonation reaction on N3, which is substantiated by the NBO negative charge decrease, for both phases, on N3 [qgN3 = -0.267 a.u. (15) → -0.187 a.u. (16)], while in protein environment the exogenous O2 is protonated [qeO2 = -0.510 a.u. (15) → -1.024 a.u. (16)].
Conclusion
Several possible pathways have been investigated for the oxidation of [Fe-Fe]-hydrogenase H-cluster, and they all proceed spontaneously to cluster, 9. Each pathway is initiated by an intermediate (1, 5, 6, and 8) of the catalytic cycles in H2 metabolism.
In gas phase, O2 binding is endergonic for the fully oxidized H-cluster 1 and exergonic for 8; however, it is exergonic for the partially oxidized 5 and reduced 6 clusters. But for aqueous enzyme phase, the O2 binding is exergonic for all oxidation states. This suggests that the fully oxidized state of the H-cluster 1 in enzyme environment is more sensitive to O2 inhibition.
Our calculations show that in the protein environment (Figure 2, and 3) the hydroxylated H-cluster 14, which is the end product of hydrogenase inhibition*, is obtained from 9 via the fully exergonic reaction pathway that starts by means of protonation (Figure 2). Antithetically, the reaction pathway that is initiated by means of reduction (Figure 3, aqueous enzyme phase) does not proceed to the hydroxylated H-cluster 14 due to this very endergonic step (ΔGQM/MM = +25.6 kcal/mol ).
The inhibitory steps in gas phase (Figure 2, and 3) consist of water removal from a closed shell, 10, and an open shell, 16, H-cluster (ΔGgas = +3.3 and +1.3 kcal/mol, respectively), while in the aqueous enzyme phase there is one inhibitory step, i.e., an e- transfer from an open shell H-cluster (9′, ΔGQM/MM = +25.6 kcal/mol ).
From gas phase geometrical analysis COb shows a displacement away from FepII (9 → 9′), but in the aqueous enzyme phase this COb translocation is not observed; the observed different phase behavior in the protein environment may be due to the imposed immobility on the iron atoms (by means of “freezing” them).
For the gas phase, cluster 11, LUMO is more localized over the Fep than on Fed, extending from the iron atoms towards the COb via a linear combination between the eg orbitals of the iron atoms with the COb π orbitals9, thus the e- transfer, 11 → 12, changes the oxidation state of Fep. However, for the protein environment, the LUMO is localized on Sγ which is being bereft of e-s via an inductive effect of the vicinal cubanes [qeSγ = 0.464 a.u. (11) vs. 0.333 a.u. (12)].
Lastly, an interesting result from the FMO gas phase analysis is that an e- is transferred to the LUMOα rather than to the virtual SOMOβ, which is rather unexpected since the virtual SOMOβ usually resides in a lower energy state than LUMOα for open-shell compounds. We also found that O2 inhibited [Fe-Fe]-hydrogenase H-cluster has OH- bonded to the Fed, and that OH- is the end product of O2 metabolism, with all aqueous enzyme phase reaction pathways proceeding exergonically.
Acknowledgments
This work was supported by funds from the Department of Energy, grant: DE-FG02-03ER15462, National Institutes of Health, grant: GM070469-01, and Cleveland State University (Established full-time faculty research development award). Computational resources have been provided by the National Center for Supercomputer Applications (University of Illinois) and the Ohio Supercomputer Center.
Footnotes
The truncation of the cubane, [Fe4-S4]2+, and its replacement by a H+ (as well as the replacement of CH3-S- for cysteine-S) had been done in order to obtain the best compromise with regard to the computational cost.
Voltametric42 studies show the transition of Hoxinact to Hoxcat occurring via a reversible e- transfer process to the hydrogenase transient state followed by a putative two e- transfer (with the latter not reaching the bimetals of the H-cluster).
Each of the three cubane/cysteine moieties (found in DdH) is comprised of a cubane plus four surrounding, depotonated cysteines which are bound to the four iron atoms of every cubane.
Where “frozen” means that the x, y, z coordinates for the atoms are kept fixed.
The similarity for these clusters is they are first found as hydride containing H-clusters, and then undergo oxidation.
The NBO charges and bond lengths are found in the supporting information addendum. qg represents the charges for the gas phase, while qe stands for the aqueous enzyme phase charges.
Which has also being confirmed by calculations (Motiu et al., unpublished results).
References
- 1.Das D, Dutta T, Nath K, Kotay SM, Das AK, Veziroglu TN. Curr Sci. 2006;90:1627. [Google Scholar]
- 2.Liu ZP, Hu P. J Am Chem Soc. 2002;124:5175. doi: 10.1021/ja0118690. [DOI] [PubMed] [Google Scholar]
- 3.Vignais PM, Billoud B, Meyer J. FEMS Microbiol Rev. 2001;25:455–501. doi: 10.1111/j.1574-6976.2001.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 4.Melis A, Zhang L, Forestier M, Ghirardi ML, Seibert M. Plant Physiol. 2000;122:127. doi: 10.1104/pp.122.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albracht SPJ. Biochim Biophys Acta. 1994;1118:167. doi: 10.1016/0005-2728(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 6.Adams MWW. Biochim Biophys Acta. 1990;1020:115. doi: 10.1016/0005-2728(90)90044-5. [DOI] [PubMed] [Google Scholar]
- 7.Adams MWW, Stiefel EI. Science. 1998;282:1842. doi: 10.1126/science.282.5395.1842. [DOI] [PubMed] [Google Scholar]
- 8.Happe RP, Roseboom W, Pierik AJ, Albracht SP, Bagley KA. Nature. 1997;385:126. doi: 10.1038/385126a0. [DOI] [PubMed] [Google Scholar]
- 9.Liu ZP, Hu P. J Chem Phys. 2002;117:8177. [Google Scholar]
- 10.Bruschi M, Fantucci P, De Gioia L. Inorg Chem. 2002;41:1421. doi: 10.1021/ic010770r. [DOI] [PubMed] [Google Scholar]
- 11.Bruschi M, Fantucci P, De Gioia L. Inorg Chem. 2003;42:4773. doi: 10.1021/ic0262132. [DOI] [PubMed] [Google Scholar]
- 12.Bruschi M, Fantucci P, De Gioia L. Inorg Chem. 2004;43:3733. doi: 10.1021/ic035326y. [DOI] [PubMed] [Google Scholar]
- 13.Zampella G, Bruschi M, Fantucci P, Razavet M, Pickett CJ, De Gioia L. Chem Eur J. 2005;11:509. doi: 10.1002/chem.200400442. [DOI] [PubMed] [Google Scholar]
- 14.Cao Z, Hall MB. J Am Chem Soc. 2001;123:3734. doi: 10.1021/ja000116v. [DOI] [PubMed] [Google Scholar]
- 15.Fan HJ, Hall MB. J Am Chem Soc. 2001;123:3828. doi: 10.1021/ja004120i. [DOI] [PubMed] [Google Scholar]
- 16.Greco C, Bruschi M, De Gioia L, Ryde U. Inorg Chem. 2007;46:5911. doi: 10.1021/ic062320a. [DOI] [PubMed] [Google Scholar]
- 17.Trohalaki S, Pachter R. ENERG FUEL. 2007;21:2278. [Google Scholar]
- 18.Greco C, Bruschi M, Heimdal J, Fantucci P, De Gioia L, Ryde U. Inorg Chem. 2007;46:7256. doi: 10.1021/ic701051h. [DOI] [PubMed] [Google Scholar]
- 19.Greco C, Bruschi M, Fantucci P, De Gioia L. Eur J Inorg Chem. 2007;13:1835. doi: 10.1021/ic8006298. [DOI] [PubMed] [Google Scholar]
- 20.Greco C, Zampella G, Bertini L, Bruschi M, Fantucci P, De Gioia L. Inorg Chem. 2007;46:108. doi: 10.1021/ic061168+. [DOI] [PubMed] [Google Scholar]
- 21.Motiu S, Dogaru D, Gogonea V. Int J Quantum Chem. 2007;107:1248. doi: 10.1002/qua.21875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruschi M, Zampella G, Fantucci P, De Gioia L. Coord Chem Rev. 2005;15-16:1620. [Google Scholar]
- 23.Liu X, Ibrahim SK, Tard C, Pickett CJ. Coord Chem Rev. 2005;15-16:1641. [Google Scholar]
- 24.Armstrong FA. Curr Opin Chem Biol. 2004;8:133. doi: 10.1016/j.cbpa.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Rauchfuss TB. Inorg Chem. 2004;43:14. doi: 10.1021/ic0343760. [DOI] [PubMed] [Google Scholar]
- 26.Evans DJ, Pickett CJ. Chem Soc Rev. 2003;35:268. doi: 10.1039/b201317g. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, Lemon BJ, Huang S, Swartz DJ, Peters JW, Bagley KA. Biochemistry. 2002;41:2036. doi: 10.1021/bi011510o. [DOI] [PubMed] [Google Scholar]
- 28.Horner DS, Heil B, Happe T, Embley TM. Trends Biochem Sci. 2002;27:148. doi: 10.1016/s0968-0004(01)02053-9. [DOI] [PubMed] [Google Scholar]
- 29.Nicolet Y, Cavazza C, Fontecilla-Camps JC. J Inorg Biochem. 2002;1 [Google Scholar]
- 30.Lyon EJ, Georgakaki IP, Reibenspies JH, Darensbourg MY. Angew Chem, Int Ed. 1999;38:3178. [PubMed] [Google Scholar]
- 31.Nicolet Y, Piras C, Legrand P, Hatchikian EC, Fontecilla-Camps JC. Structure. 1999;7:13. doi: 10.1016/s0969-2126(99)80005-7. [DOI] [PubMed] [Google Scholar]
- 32.Cloirec AL, Best SP, Borg S, Davies SC, Evans DJ, Hughes DL, Pickett CJ. Chem Commun. 1999:2285. [Google Scholar]
- 33.Rauchfuss TB, Contakes SM, Schmidt M. J Am Chem Soc. 1999;121:9736. [Google Scholar]
- 34.Lai CH, Lee WZ, Miller ML, Reibenspies JH, Darensbourg DJ, Darensbourg MY. J Am Chem Soc. 1998;120:10103. [Google Scholar]
- 35.Pierik AJ, Hulstein M, Hagen WR, Albracht SP. Eur J Biochem. 1998;258:572. doi: 10.1046/j.1432-1327.1998.2580572.x. [DOI] [PubMed] [Google Scholar]
- 36.Pierik AJ, Hagen WR, Redeker JS, Wolbert RBG, Boersma M, Verhagen MF, Grande HJ, Veeger C, Mustsaers PHA, Sand RH, Dunham WR. Eur J Biochem. 1992;209:63. doi: 10.1111/j.1432-1033.1992.tb17261.x. [DOI] [PubMed] [Google Scholar]
- 37.Zambrano IC, Kowal AT, Mortenson LE, Adams MWW, Johnson MK. J Biol Chem. 1989;264:20974. [PubMed] [Google Scholar]
- 38.Patil DS, Moura JJG, He SH, Teixeira M, Prickril BC, Der Vartanian DV, Peck HD, Jr, Legall J, Huynh BH. J Biol Chem. 1988;263:18732. [PubMed] [Google Scholar]
- 39.Rusnak FM, Adams MWW, Mortenson LE, Munck E. J Biol Chem. 1987;262:38. [PubMed] [Google Scholar]
- 40.Adams MWW. J Biol Chem. 1987;262:15054. [PubMed] [Google Scholar]
- 41.Adams MWW, Mortenson LE. J Biol Chem. 1984;259:7045. [PubMed] [Google Scholar]
- 42.Roseboom W, De Lacey AL, Fernandez VM, Hatchikian EC, Albracht SPJ. J Biol Inorg Chem. 2006;11:102. doi: 10.1007/s00775-005-0040-2. [DOI] [PubMed] [Google Scholar]
- 43.Peters JW, Lanzilotta WN, Lemon BJ, Seefeldt LC. Science. 1998;282:1853. doi: 10.1126/science.282.5395.1853. [DOI] [PubMed] [Google Scholar]
- 44.Rappe AK, Casewit CJ, Colwell KS, Goddard WA, III, Skiff WM. J Am Chem Soc. 1992;113:10024. [Google Scholar]
- 45.Dapprich S, Komaromi I, Byun KS, Morokuma K, Frisch MJ. J Mol Struct (Theochem) 1999;1:461. [Google Scholar]
- 46.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JJA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, L X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, D AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Gaussian 03, Revision C.02. Gaussian, Inc.; Wallingford CT: 2004. [Google Scholar]
- 47.Becke AD. J Chem Phys. 1993;98:5648. [Google Scholar]
- 48.Lee C, Yang W, Parr RG. Phys Rev B. 1988;37:785. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 49.Hay PJ, Wadt WR. J Chem Phys. 1985;82:270. [Google Scholar]
- 50.Wadt WR, Hay PJ. J Chem Phys. 1985;82:284. [Google Scholar]
- 51.Berendsen HJC, van der Spoel D. Comp Phys Comm. 1995;91:43. [Google Scholar]
- 52.Lindahl E, Hess B. J Mol Mod. 2001;7:306. [Google Scholar]
- 53.Popescu CV, Munck E. J Am Chem Soc. 1999;121:7877. [Google Scholar]
- 54.Rappe AK, Goddard WA., III J Phys Chem. 1991;95:3358. [Google Scholar]
- 55.Peters JW. Curr Opin Struct Biol. 1999;9:670. doi: 10.1016/s0959-440x(99)00028-7. [DOI] [PubMed] [Google Scholar]
- 56.George SJ, Kurkin S, Thorneley RNF, Albracht SPJ. Biochemistry. 2004;43:6808. doi: 10.1021/bi049853k. [DOI] [PubMed] [Google Scholar]