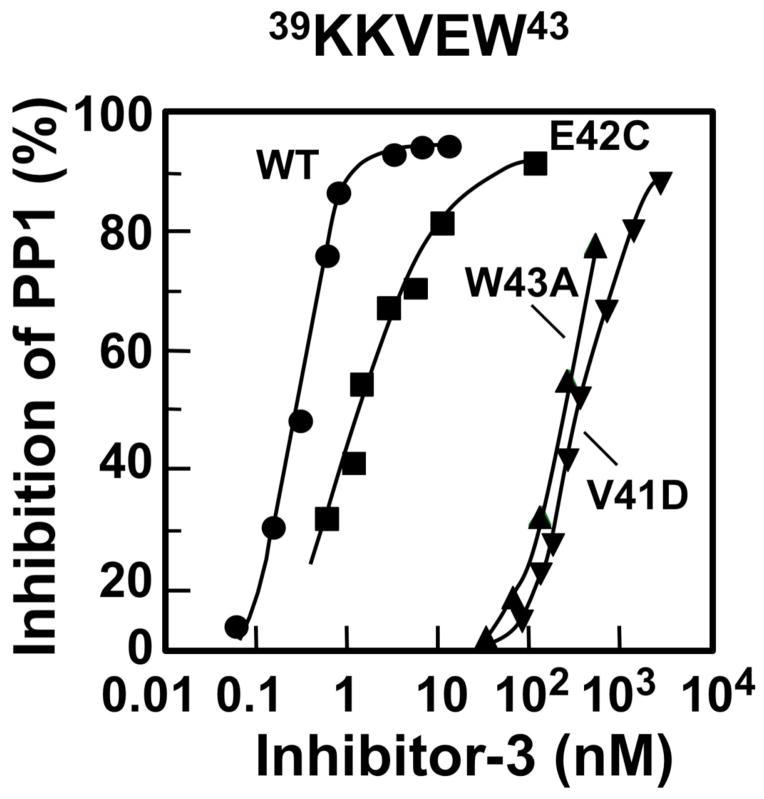
Figure 1. Effects of mutations of the KKVEW sequence of Inh3 on the inhibition of PP1 activity.
The V41D, E42C, W43A mutants of Inh3 were purified to near-homogeneity and their abilities to inhibit PP1 activity were determined as a function of concentration (Experimental Procedures). Results were plotted as percentage of inhibition of phosphorylase phosphatase activity: wild type Inh3, circles; Inh3-E42C, squares; Inh3-W43A, triangles; Inh3-V41D, inverted triangles.