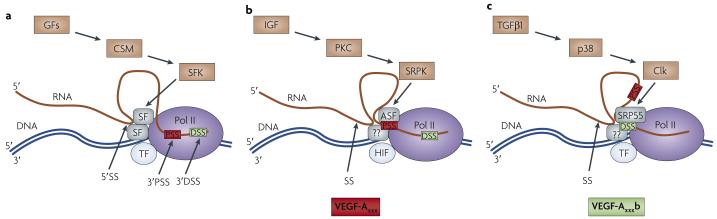
Figure 2. Vascular endothelial growth factor A (VEGF-A) C’ terminal splicing regulation.
a | The C’ terminal domain of RNA polymerase II (Pol II) interacts with both transcription factors (TFs) and splicing factors (SFs). SFs are recruited to the transcriptional machinery owing to their interaction with Pol II72,73. These SFs recognize cis-acting RNA splicing sequences in the pre-mRNA and both splicing sites (SS) — 5′ donor (5′SS) or 3′ acceptor sites — can be recognized. Both 3′ proximal SS (3′PSS) and distal SS (3′DSS) are indicated. The particular splicing factors recruited are dependent on the sequence. These SFs can be regulated by SF kinases (SFKs), which are regulated by cell signalling molecules (CSMs) downstream of growth factors (GFs). b | Regulation of VEGF-A C’ terminal PSS selection by insulin-like growth factor (IGF). IGF activates protein kinase C (PKC), which results in phosphorylation of SR protein kinases (SRPKs). These can activate the ASF-SF2 splicing factor, which favours PSS selection. This process may be dependent on the presence of hypoxia-inducible factor (HIF), a transcription factor involved in VEGF-Axxx upregulation13. Other SFs and kinases may also be involved in PSS and DSS selection, denoted by ‘??’. c | Factors affecting VEGF-A C’ terminal DSS selection. Transforming growth factor β1 (TGFβ1) results in p38 mitogen-activated protein kinase activation and subsequent activation of the kinases CLK1 and CLK4. CLK1 and CLK4 phosphorylate the splicing factor SRP55, resulting in DSS selection and production of VEGF-Axxxb13. It is also possible that ASF-SF2 is inactivated by CLK1 and CLK4, or that phosphorylation of the SFs could change their location, degradation or binding affinity. This scheme summarizes the limited data available.