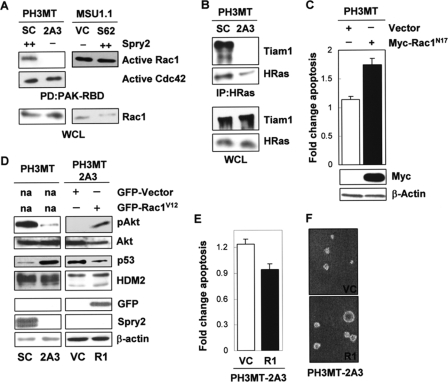
FIGURE 4.
Effect of Spry2 on Rac1 activation in HRas-transformed cells. A, WCL from the HRas-transformed cell strain expressing a high endogenous level of Spry2 (PH3MT-SC (SC)) or a reduced level of Spry2 (PH3MT-2A3 (2A3)) and the parental non-transformed human fibroblast cell strain expressing a low endogenous level of Spry2 (empty vector) (MSU1.1-VC (VC)) or expressing a high level of Spry2 (MSU1.1-S62 (S62)) were pulled down (PD) with PAK-CRIB-conjugated beads. The amount of Rac1 bound to the beads, as well as the Rac1 present in the WCL was determined. The amount of active Cdc42 was determined only in the PH3MT cells. B, WCL from PH3MT-SC and PH3MT-2A3 cell strains were immunoblotted or immunoprecipitated (IP) with an antibody specific to HRas and then immunoblotted to detect Tiam1 and HRas with the indicated antibodies. C, HRas-transformed fibroblasts (PH3MT) were stably transfected with an empty vector or a vector encoding a Myc-tagged, dominant negative form of Rac1 (Rac1N17). WCL from these stable clones were analyzed by Western blotting for Rac1 and β-actin expression. The same cell strains were treated and analyzed as in Fig. 1A. The average of two independent experiments is shown. D, HRas-transformed cells with down-regulated Spry2 (PH3MT-2A3) but stably expressing GFP-Rac1V12 (2A3-R1) or GFP alone (2A3-VC) were analyzed by Western blotting for pAkt; Akt; p53; HDM2; GFP; Spry2; or β-actin. E, the PH3MT-2A3 VC and PH3MT-2A3 R1 cell strains were UV-irradiated and analyzed as in Fig. 1A. The average of three independent experiments is shown. F, the indicated cell strains (5,000 cells/dish) were grown in agarose in a culture medium containing 2.5% serum for 3 weeks, as described in Ref. 31. Representative pictures of the colonies that formed in agarose are shown. R1, PH3MT-2A3-R1.