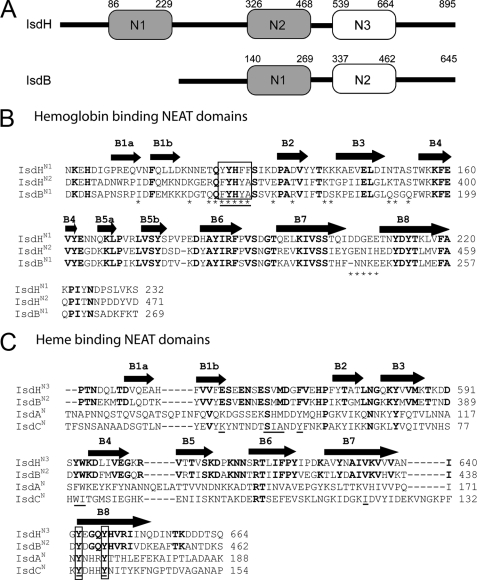
FIGURE 1.
Comparison of the NEAT domains in S. aureus. A, schematic of the IsdB and IsdH proteins showing that they contain two and three NEAT domains, respectively. Domains are shaded based on their relatedness. Domains shaded gray share 46-65% primary sequence identity (IsdHN1, IsdHN2, and IsdBN1). Non-shaded domains share 49% primary sequence identity (IsdHN3 and IsdBN2). Based on studies of the isolated NEAT domains within IsdH, the gray- and non-shaded domains bind Hb and heme, respectively. Both proteins also contain a cell wall sorting signal motif at their C termini that is covalently attached to the cell wall by the SrtA sortase enzyme. B, sequence alignment of related Hb binding NEAT domains within IsdH and IsdB: IsdHN1, IsdHN2, and IsdBN1. These domains are closely related to one another and share 46-65% sequence identity. Residues mutated in this study are indicated with an asterisk, and amino acids are enclosed in a box if their mutation to alanine completely disrupts Hp binding and/or reduces the affinity of IsdHN1 for Hb by at least ×50-fold. The aromatic motif diagnostic of NEAT domains that bind Hb is underlined. The secondary structure of IsdHN1 is shown above the primary sequence. Completely conserved residues are in bold. Only the IsdHN1 and IsdHN2 domains have thus far been shown to bind Hb, whereas the IsdBN1 domain alone has yet to be tested. C, sequence alignment of the IsdHN3 and IsdBN2 NEAT domains, which share 49% sequence identity. The sequences of the distantly related IsdA and IsdC domains are also shown because they bind to heme. Positions within the primary sequence that are within 3.5 Å of the heme in the NMR and crystal structures of the IsdC-heme and IsdA-heme complexes are underlined. The invariant tyrosine residues that coordinate the iron atom of the heme in these structures is enclosed in a box. Residues that are completely conserved in IsdHN3 and IsdBN2 are in bold.