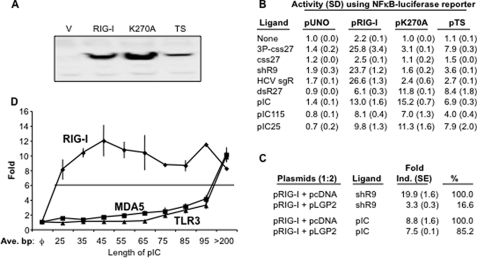
FIGURE 1.
Single- and double-stranded ligands have different properties for RIG-I signaling. A, Western blot analysis of RIG-I and mutants. RIG-I protein and the two alanine substitution mutants are expressed in transiently transfected 293T cells. The Western blot was probed with a polyclonal antibody against C-terminal region of RIG-I purchased from Santa Cruz Biotechnology, Inc. V, vector. B, the effects of two RIG-I mutations on response to different ligands. Numbers denote -fold induction that is the ratio of the induced versus uninduced samples assayed. The assay was performed using luciferase reporter regulated by a promoter containing NF-κB binding sites. Each number represents the mean of at least three independent assays, and the value of 1 S.E. is shown in parentheses. Throughout this work the following names are used for the various ligands; 3P-css27 is a 27-nt RNA produced by in vitro transcription, and css27 is the same RNA that lacks 5′-triphosphates. ShR9 is a 60-nt hairpin RNA produced by in vitro transcription (see supplemental Fig. 1). HCV sgR is the hepatitis C virus subgenomic replicon RNA transcribed from linearized plasmid pFK/I389neo/NS3-3′/5.1. DsR27 is a double-stranded RNA made by annealing two single-stranded oligonucleotides of 27-nt each; pIC is poly(I:C) purchased from Invitrogen and has a molecular mass that is in excess of 200 bp with very little fragments below this length. pIC115 and pIC25 are poly(I:C)s of 115 and 25 bp, respectively. C, effects of shR9-induced RIG-I activity in the presence of LGP2. The ratio of RIG-I to LGP2 is 1:2. D, the effects of poly(I:C) length on induction of RIG-I activation of an NF-κB reporter in HEK293T cells. The same series of pIC was used to examine MDA5 and TLR3 signaling and yielded different responses. Each point is the graph shows the mean and S.E. of three independent assays.