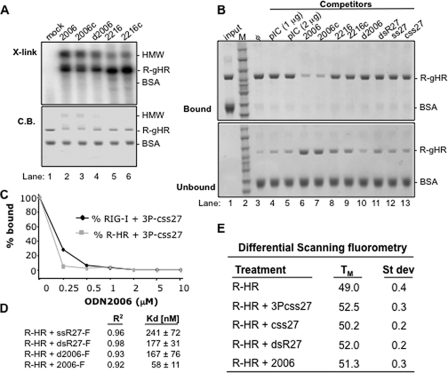
FIGURE 4.
Properties of antagonist binding to RIG-I or its derivatives. A, results from a UV cross-linking assay demonstrating that purified R-gHR can be cross-linked to ODNs. The top panel is an autoradiogram of the cross-linked products in a SDS-PAGE. The bottom panel is the SDS-PAGE stained to reveal the locations of the proteins used in the reaction. BSA is used as an internal negative control in all of the reactions. HMW denotes an oligomeric form of RIG-I that is preferentially detected with strong antagonists. B, results of a pulldown assay examining competition of different ligands with R-gHR. The assay uses biotinylated 2006 bound to streptavidin resin, which can specifically bind R-gHR, but not the internal specificity control, BSA. The binding assay was performed in the presence of various competing ligands shown on the top of the gel. C, a competition assay examining UV cross-linking to a internally labeled 5′-triphosphorylated RNA in the presence of increasing concentrations of antagonist 2006. D, fluorescent anisotropy to determine affinity for ligand. Fluorescein isothiocyanate-labeled ligands were used to measure Kd values using R-HR protein, and the R2 values show the fit of the data to the binding isotherm. E, results of differential scanning fluorometry used to measure the melting point (TM) of RIG-I in the presence of different ligands. Results shown are from three independent experiments.