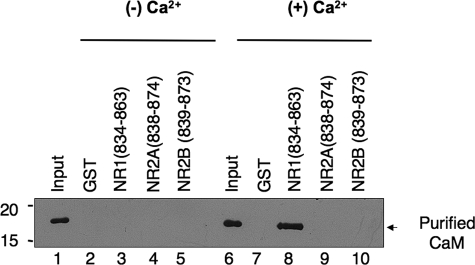
FIGURE 5.
The membrane-proximal carboxyl-terminal domains of NMDAR2A and NMDAR2B subunits do not bind calmodulin. Calmodulin is structurally related to the myosin light chains yet does not bind NR2 subunits. The membrane-proximal region of NR1 was used as a positive control; NR1-(834-863) harbors one of two calmodulin-binding sites on the carboxyl terminus of the NR1 subunit that binds calmodulin in a strictly calcium-dependent manner. NR1-(834-863), NR2A-(838-874), and NR2B-(839-873) were expressed as GST fusion proteins, immobilized on glutathione-Sepharose beads, and tested for their ability to bind calmodulin in the absence (lanes 1-5) and presence (lanes 6-10) of calcium (2 mm). Assays were initiated by the addition of 500 nm calmodulin (Calbiochem) for 2 h at 4 °C in the presence and absence of calcium. Where appropriate, calcium (2 mm) was present throughout the experiment, including all wash buffers. Calmodulin bound to GST fusion proteins was resolved by PAGE and blotted to nitrocellulose. Immune complexes were detected using an anti-calmodulin antibody (Upstate Biotechnology, Inc.). Figure is representative of three independent experiments.