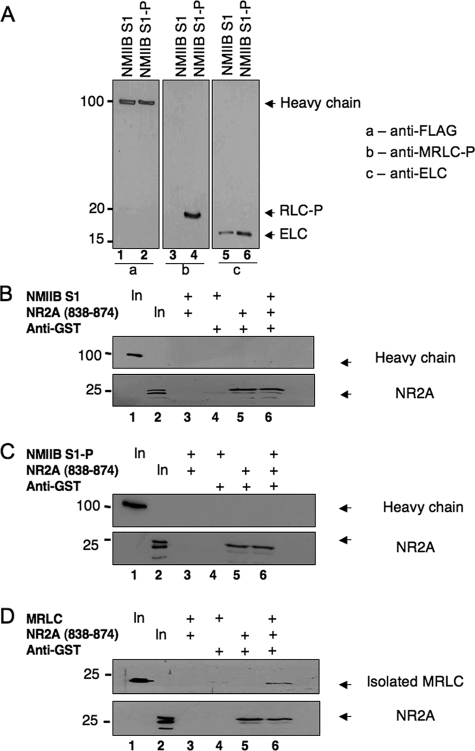
FIGURE 7.
Myosin RLC does not bind NMDAR2A in the context of either a phosphorylated or nonphosphorylated myosin II subfragment. An NMIIB S1, consisting of a truncated heavy chain in complex with RLC and ELCs, did not form a ternary complex with NR2A. A, immunoblot analysis verifying phosphorylation of NMIIB S1 (NMIIB S1-P) by MLCK. Blots were probed with antibodies to all three components of the myosin II complex and are shown as follows: panel a, lanes 1 and 2, detection of heavy chain probed with an anti-FLAG antibody (Sigma); panel B, lanes 3 and 4, detection of phosphorylated myosin RLC (RLC-P) probed with a phospho-specific myosin RLC antibody raised in the laboratory; and panel c, lanes 5 and 6, detection of myosin ELC (ELC) probed with an anti-MLC1 antibody (Abcam). B, myosin II-B heavy chain (upper panel) is not precipitated with NR2A-(838-874) as a component of a nonphosphorylated NMIIB S1. C, myosin II-B heavy chain (upper panel) is not precipitated with NR2A-(838-874) as a component of MLCK-phosphorylated NMIIB S1. D, co-immunoprecipitation of recombinant MRLC with carboxyl-terminal NR2A-(838-938). For immunoprecipitation studies, purified GST-NR2A-(838-874) was incubated with nonphosphorylated NMIIB S1 (B), phosphorylated NMIIB S1 (C), or isolated myosin RLC (D) for 1 h at 4 °C in binding buffer (10 mm HEPES (pH 7.5), 100 mm NaCl, 1 mm EDTA, 1 mm dithiothreitol, 0.1% Nonidet P-40 and 10% glycerol). Protein complexes were then incubated with an anti-GST antibody and precipitated with protein G-Sepharose. Protein complexes were resolved by PAGE, transferred to nitrocellulose, and revealed by immunoblot analysis of NR2A and each component of the myosin II complex. The phosphorylation status of NMIIB was determined after each phosphorylation reaction; B-D are representative of at least three independent determinations.