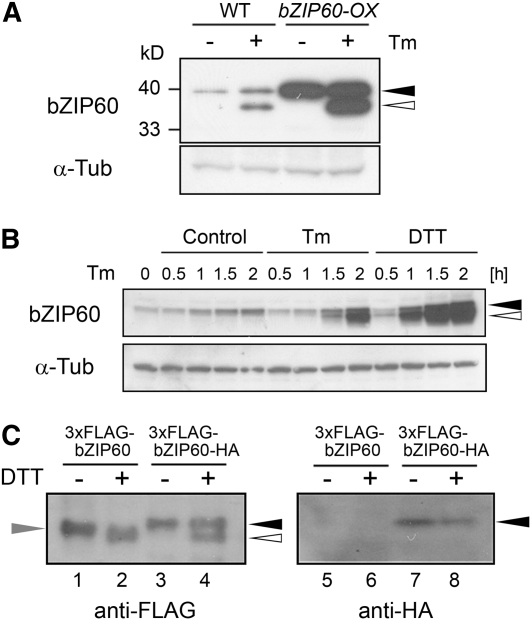
Figure 4.
Proteolytic Processing of bZIP60 in Response to ER Stress.
(A) Detection of bZIP60 protein. Total protein was extracted from wild-type and bZIP60-OX cells treated with 5 μg/mL tunicamycin (+) or 0.1% DMSO (−) for 2 h, and 20 μg of protein was subjected to immunoblot analysis using an anti-bZIP60 antibody as well as an anti-α-tubulin antibody as a loading control. Closed and open triangles in (A) and (B) indicate the positions of the full-length and faster-migrating putative cleaved forms of bZIP60, respectively. Positions of protein marker bands were indicated at the left side.
(B) Time-course analysis of bZIP60 protein by ER stress. Arabidopsis suspension cells were treated with 5 μg/mL tunicamycin (Tm), 2 mM DTT, or 0.1% DMSO (Control) at the indicated times, and total protein extracts (20 μg) were gel-fractionated and subjected to immunoblot analysis using an anti-bZIP60 antibody as well as an anti-α-tubulin antibody as a loading control.
(C) Identification of cleaved bZIP60. Arabidopsis suspension cells expressing either bZIP60 tagged with 3xFLAG at the N terminus (3xFLAG-bZIP60) or bZIP60 tagged with 3xFLAG and HA at the N and C termini, respectively (3xFLAG-bZIP60-HA) were treated with 2 mM DTT for 1 h (+) or maintained as controls (−). Total protein was extracted, and 20 μg of protein was gel-fractionated and subjected to immunoblot analysis using anti-FLAG and anti-HA antibodies. The positions of bZIP60 tagged with both 3xFLAG and HA, and bZIP60 tagged with only 3xFLAG are indicated by closed and gray triangles, and the position of the cleaved form of bZIP60, tagged with 3xFLAG at the N terminus, is indicated by the open triangle.