Table 2.
Arylation scope with respect to aryl iodidesa
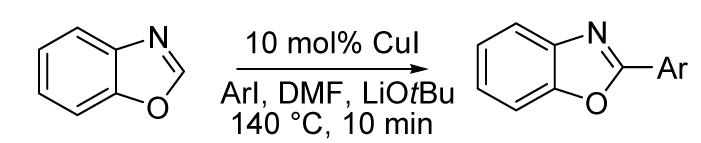 | |||
|---|---|---|---|
| Entry | ArI | Product | Yield, % |
| 1 | 4-CF3C6H4I |
|
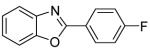
91 |
| 2 | 4-FC6H4I |
|
90 |
| 3 | 4-MeOC6H4I |
|
80 |
| 4 | 3,5-Me2C6H3I |
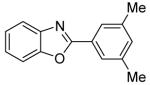
|
85 |
| 5 | 2-MeC6H4I |
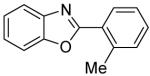
|
91 |
| 6 | 2,4,6-Me3C6H2I |
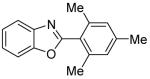
|
55 |
| 7 | 1-Iodonaphthalene |
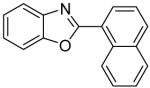
|
90 |
| 8 | 2-Iodopyridine |
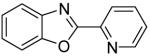
|
89 |
Substrate (1 equiv), aryl iodide (3 equiv), base (2 equiv). Yields are isolated yields.
