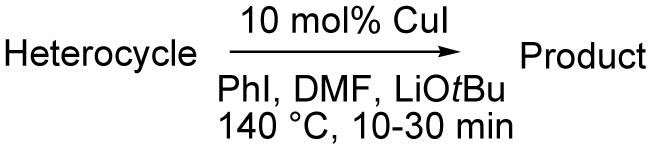
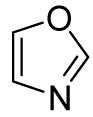
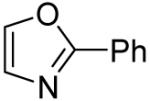
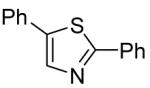
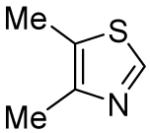
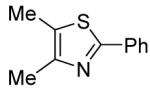
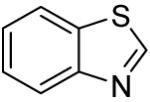
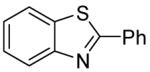
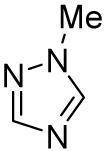
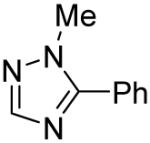
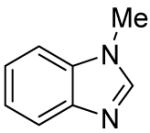
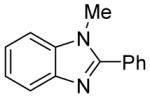
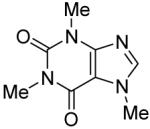
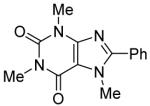
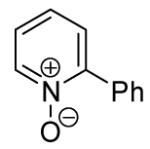
Table 3.
Arylation scope with respect to heterocyclesa
Substrate (1 equiv), iodobenzene (3 equiv), base (2 equiv). Yields are isolated yields.
2,5-Diphenyloxazole also isolated (7%).
2-Phenylthiazole also isolated (37%).
KOtBu base.
LiOtBu/KOtBu base (1:1).