Figure 3.
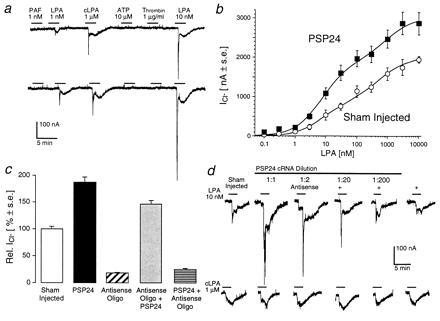
(a) Representative responses to LPA, cLPA, and the other mediators used for primer design in control (upper trace) and PSP24 cRNA-injected (0.5 ng, lower trace) oocytes from the same frog. Notice that the cLPA response is practically identical in both cells, whereas the response to LPA is 2.5- to 3-fold higher (10 nM and 1 nM, respectively). None of the other mediators triggered an oscillatory response in either cell. (b) LPA dose–response relationship in sham-injected control (open circles, n = 4) and PSP24 cRNA-injected (solid squares, n = 5) oocytes. The responses in PSP24 cRNA-expressing oocytes exceeded those of the controls and showed an even more pronounced enhancement between 0.1 and 100 nM, contributing to the high-affinity receptor for LPA. (c) An antisense oligonucleotide designed from the sequence of amino acids 5–11 of clone PSP24 inhibits both the endogenous and the induced LPA response (n = 6 for all conditions). Injection of PSP24 cRNA at 0.5 ng per oocyte (solid bar) approximately doubled the response to 10 nM LPA in this experiment. Injection of ≈0.3 fmol (2.5 pg) of the 18-mer antisense oligonucleotide (hatched bar) reduced the endogenous response by 80%. To avoid the injection of double-stranded RNA that triggers the activation of nucleases, oocytes were injected a second time 2 days after the first injection with either PSP24 cRNA or the antisense oligonucleotide. A second injection of PSP24 cRNA (0.5 ng) into the oocytes (shaded bar) that were first injected with antisense oligonucleotide caused an ≈1.5-fold increase in the mean response over that of the distilled water-injected (“sham-injected”) controls. A second injection of the antisense oligonucleotide into oocytes that were first injected with PSP24 cRNA inhibited the response 73% below the endogenous response. All bars represent the mean response (±SEM) for six oocytes. (d) The increase in the LPA response is selective and dependent on the amount of PSP24 cRNA present. In this experiment, oocytes were injected with a 1- to 200-fold dilution of a stock solution of PSP24 cRNA (1 μg/μl, 50 nl per oocyte). A constant amount (0.3 fmol) of antisense cRNA was mixed with and coinjected with increasing dilutions of sense cRNA. Responses to 10 nM LPA and 1 μM cLPA were measured 4–6 days after injection. Traces are representative of four independent determinations (n = 4). Note that the increase in the LPA response decreased with the decreasing amount of PSP24 cRNA injected, whereas the cLPA response was virtually unaffected in all groups. Injection of 0.3 fmol of antisense cRNA, alone, caused a 45% reduction in the endogenous LPA response, whereas the cLPA response was virtually unaltered.
