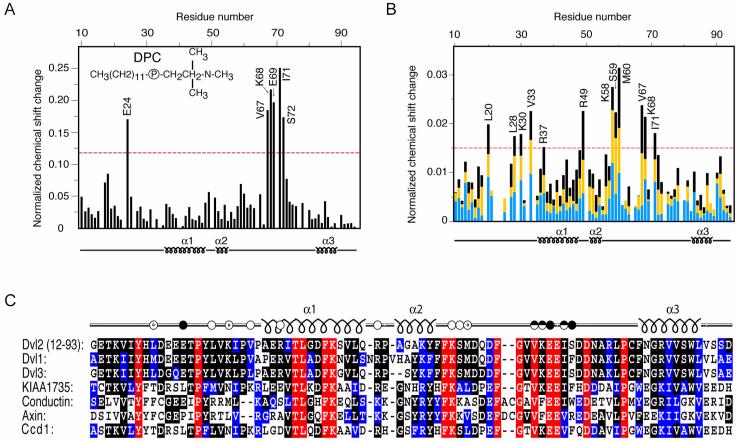
Fig. 4.
Identification of ligand-interacting residues of the DIX domain by HSQC experiments. (A) The histogram displays the normalized chemical shift difference for each residues amide group of 15N-labeled Dvl2 DIX domain (100 μM) caused by increasing the d38-DPC concentration from 10 to 400 mM. (B) Histogram showing the progressive changes in chemical shifts of DIX domain (100 μM) residues following actin addition. Blue, yellow and black bars represent reductions after addition of 200, 300, and 400 μM G-actin, respectively. The 1H,15N chemical shifts were normalized as ([(ΔδH)2 + (ΔδN/5)2]/2)0.5, where δ is chemical shift in parts per million (ppm). Labeled residues exhibit changes that exceed the red line, which represents 50% of the largest change. (C) The amino acid sequences of the DIX domains from human Dvl homologs, KIAA1735, Conductin and Axin and zebrafish Ccd1 are aligned. Identical, highly conserved and similar residues are shown in red, black and blue, respectively. The Dvl2 residues that are involved in actin and DPC micelle binding are shown with unfilled and filled dots, respectively.