Fig. 1.
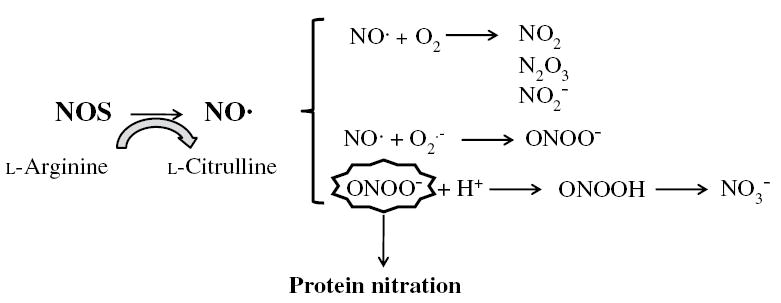
Generation of NO· by NOS in liver cells. NO· is relatively unstable in the presence of O2 and will rapidly and spontaneoulsy auto-oxidize to yield a variety of nitrogen oxides.NO· also reacts with O2· − to generate ONOO−. Although ONOO− is relatively stable, it has a pKa of 6.8, which implies that substantial amounts of ONOO− will be protonated at physiologic pH to yield peroxynitrous acid. This conjugate acid rapidly decomposes to yield NO3−. Nitration of tyrosine residues by ONOO− forms the stable product, 3-nitrotyrosine (3-NT, the footprint for ONOO−) by addition of a nitro group to the 3-position adjacent to the hydroxyl group of tyrosine.
