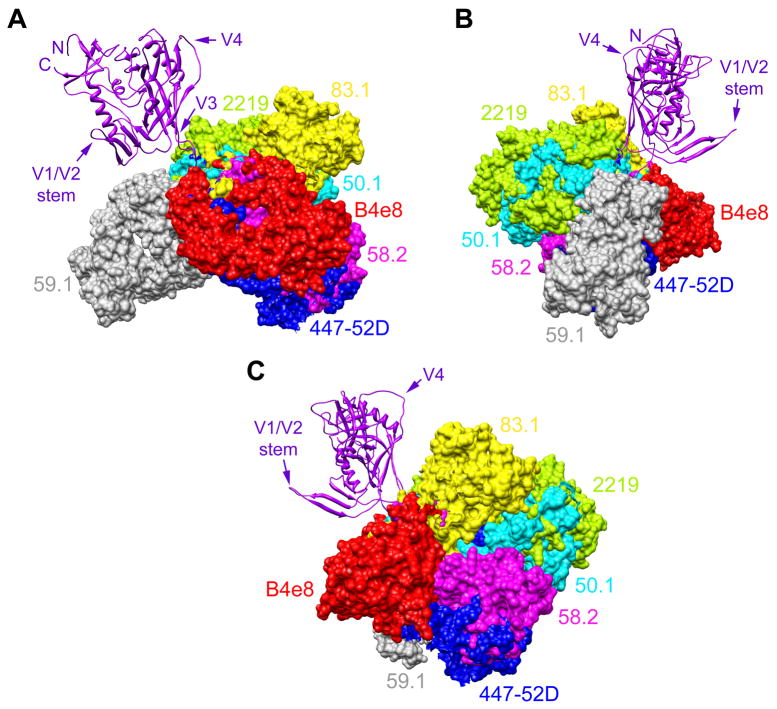
Fig. 3.
Angle of interaction between V3 antibodies and V3. Structure of gp120JR-FL-core containing V3 (Huang et al., 2005) (purple; ribbon representation) shown from 3 perspectives with the Fab fragments of 7 different mAbs (surface rendering) superimposed to depict their different angles of interaction with V3. (A) Gp120 from the perspective of CD4; (B) Gp120 from the perspective of gp41 and the gp120:gp41 interface; (C) Gp120 from the approximate perspective of a coreceptor molecule (e.g. CCR5). To establish the angle for each mAb, the V3 peptide (not shown for clarity purposes) from each mAb:peptide complex structure was superimposed onto the V3 structure of gp120 using the MatchMaker feature in UCSF Chimera, which constructs a sequence alignment and then performs a least-squares fit to superimpose the aligned residue pairs; the sequence alignment is based on a combination of residue identity/similarity and secondary structure correspondence. The V3 mAbs shown are: B4e8 (red), 447-52D (blue), 58.2 (magenta), 59.1 (grey), 50.1 (cyan), 83.1 (yellow), and 2219 (green). Aside from V3, additional regions of gp120 (N- and C termini, V1/V2 stem, and V4) are denoted to orientate the reader.