Figure 3.
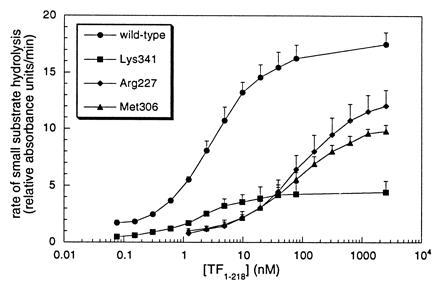
Effect of Ala substitutions of VIIa protease domain residues on amidolytic function of TF·VIIa. The rate of hydrolysis of Chromozym tPA by 5 nM mutant or wild-type VIIa is shown versus the concentration of soluble TF1–218. Binding defects are apparent for mutations of Arg-277 [c134] (KDapp = 73 ± 1 nM) and Met-306 [c164] (KDapp = 77 ± 16 nM), as compared with wild-type VIIa (KDapp = 2.8 ± 0.3 nM) and the mutant at residue Lys-341 [c192] (KDapp = 1.7 ± 0.6 nM).
